Coronary Artery Origins
Surprise Origin for Coronary Arteries Could Speed Advances in Regenerative Medicine
November 21, 2012 — (BRONX, NY) — During embryonic development, the all-important coronary arteries arise from cells previously considered incapable of producing them, according to scientists at Albert Einstein College of Medicine of Yeshiva University. The research, carried out in mice and published today in the online edition of the journal Cell, may speed development of regenerative therapies for heart disease.

Bin Zhou, M.D., Ph.D.Each year, more than one million Americans undergo coronary revascularization which includes coronary artery bypass graft (CABG). During CABG, doctors remove a portion of a healthy vein, usually from a patient’s leg, then bypass diseased areas of the coronary arteries. While the procedure has become routine and is considered relatively safe and long-lasting, the veins used during bypass do not completely mimic the arteries they bypass. They can sometimes re-clog, a process known as restenosis, requiring further procedures. Therefore, the ability to regenerate coronary arteries could usher in a new wave of more effective cardiac care.
Coronary arteries nourish heart muscle with the nutrients and oxygen it needs for pumping. Heart attacks occur when coronary arteries become blocked, causing heart muscle to die. Recent studies had suggested that during development, the coronary arteries originate from cells of the sinus venosus (a heart cavity that exists only in embryos) or from the epicardium (the heart’s outermost layer).
In their study, Einstein scientists used a wide variety of research tools to show that the coronary arteries largely arise from cells of the endocardium, the heart’s innermost cell layer. In particular, the arteries arise from endocardial cells lining the ventricles (the two large chambers of the heart).
"The prevailing wisdom was that endocardial cells are terminally differentiated, meaning they cannot become any other cell type," said study leader Bin Zhou, M.D., Ph.D., associate professor of genetics, of pediatrics, and of medicine at Einstein. "But our study shows that one population of endocardial cells is actually responsible for forming the coronary arteries."
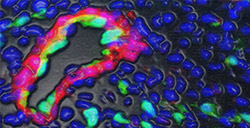
Dr. Zhou and colleagues found that ventricular endocardial cells are progenitors of the coronary arteries. This illustration shows daughter cells of ventricular endocardial cells that have formed the inner endothelium (in green) of a mature major coronary artery.More specifically, ventricular endocardial cells develop into coronary artery progenitor (precursor) cells, which then go on to form the coronary arteries. Dr. Zhou and his colleagues also identified a key signaling pathway involved in transforming the ventricular endocardial cells into coronary artery progenitor cells. Einstein has filed a patent application related to this research. The Nfatc1 cell technology is available for licensing.
The Einstein researchers are now trying to identify all the signaling mechanisms that guide the development of the coronary arteries, with the aim of one day synthesizing healthy coronary arteries to replace diseased ones. "When provided with the right environmental signals, vascular progenitor cells can form functional vessels in a petri dish," said Dr. Zhou. "If we can figure out the critical signals regulating coronary artery differentiation and formation, then perhaps we could coax ventricular endocardial cells to build new coronary arteries that can replace damaged ones—basically duplicating the way that these vessels are formed in the body," said Dr. Zhou.
The paper is titled, "Endocardial Cells Form the Coronary Arteries by Angiogenesis through Myocardial-Endocardial VEGF Signaling." Other Einstein contributors are Bingruo Wu, M.D.; Zheng Zhang, Ph.D.; Wendy Lui, B.S.; Xiangjian Chen, M.D., PhD.; Yidong Wang, Ph.D.; Alyssa Chamberlain, B.S.; Brian P. O’Rourke, B.S.; David J. Sharp, Ph.D.; Deyou Zheng, Ph.D.; and Jack Lenz, Ph.D. Other contributors include Ricardo A. Moreno-Rodriguez, Ph.D. and Roger R. Markwald, Ph.D., at Medical University of South Carolina, Charleston, SC; H. Scott Baldwin, M.D. at Vanderbilt University, Nashville, TN; and Ching-Pin Chang, M.D., Ph.D., at Stanford University School of Medicine, Stanford, CA.
The study, initiated at Vanderbilt University and completed at Albert Einstein College of Medicine, was supported by grants from the National Institutes of Health (HL078881 to Dr. Zhou, HL100398 to H. Scott Baldwin, and HL85345).
Other Top Stories
9/11 World Trade Center Exposure Linked to Heart Disease Among NYC Firefighters
On Becoming a Physician: New Einstein Students Receive White Coats and Stethoscopes
Novel Therapy for Acute Migraine Shows Promise in Phase 3 Clinical Trial
First Complete Wiring Diagram of an Animal's Nervous System
Multimillion Dollar NIH Grant to Help Reduce Opioid Use & Get Care to People Who Need It
NIH Grant Funds $23 Million Study of Diseases Affecting People Living with HIV
New TAILORx Data Guides Adjuvant Therapy in Younger Breast Cancer Patients
Einstein Celebrates Its 61st Commencement
Bolstering Biopsies: Testing Patients' Individual Cells to Guide Treatment



Tablet Blog