 |
The Casadevall Laboratory is interested in two fundamental questions: 1) How do microbes cause diseases; 2) How do hosts protect themselves against microbes? To address these broad questions the laboratory takes multidisciplinary approaches that span several areas of basic immunology and microbiology. Our research mainly focus on pathogenic microbes including Cryptococcus neoformans, Bacillus anthracis, and Mycobacterium tuberculosis. Some of our scientific interests are described below.
Cryptococcus neoformans
C. neoformans is the causative agent of cryptococcosis, affecting mostly immunocompromised patients, especially those with HIV infection, cancers, and organ transplant. The three well established virulence factors of C. neoformans include the capsule, melanin, and the ability to survive at human body temperature. Antifungal drugs are commonly used to treat fungal infections, but the problem of emerging drug-resistant strains has become more serious. Therefore, a better prevention and treatment such as vaccine is urgently needed.
Capsule structure and function
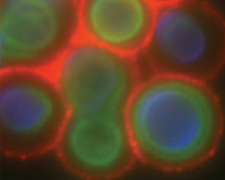
The capsule, consisting of glucuronoxylomannan (GXM), galactoxylomannan (GalXM), and mannoprotein, is one of the main virulence factors of C. neoformans. The size, structure, and physical property of the capsule can dramatically change depending on the growth environment, age of culture, and serotype. We study the composition and physical properties of GXM and GalXM in order to better understand the macromolecular structure of the capsule and to resolve key issues such as the identity of polysaccharide epitopes recognized by protective antibodies. We are also interested in the process of capsule growth, and how this capsule dynamics being translated into virulence against the host.
Melanin structure and function
Melanin production in C. neoformans is associated with virulence. Melanin is a pigment with an undefined chemical structure and tremendous physical stability. This pigment accumulates in the cell wall of C. neoformans. We are interested in understanding the fundamental biological process of how melanin in the cell wall is remodeled to allow growth and budding to occur. In addition, we are also studying how bacterial-fungal interactions, which may occur in the environment, cause melanization of C. neoformans, ultimately allowing resistance against amoeboid predators and mammalian hosts.
Antibody structure and binding specificity
The structure of the constant region of an antibody molecule can influence the function of the variable region including its binding specificity. We use a variety of spectroscopic techniques including X-ray crystallography to probe the structural and electronic properties of the constant region of the antibody against GXM, in order to reveal how conformational differences in antibody isotypes influence the binding specificity.
Quorum sensing
Quorum Sensing (QS) is a mechanism of communication between microbial cells, mediated by molecules (QSM) that are accumulated during cell growth. When the QSMs reach a certain threshold concentration, they induce the entire population to cooperate in behaviors such as bioluminescence, antibiotic production, sporulation, biofilm formation, and virulence. QS is well known in bacteria, but eukaryotic QS was unknown until the recent discovery of QSMs in Candida albicans. We have thus decided to investigate the presence of cell density-dependent behavior in C. neoformans and to check the possible effects of cell-density molecules in growth, virulence, and host-pathogen interactions.
Biofilm formation
Biofilms are communities of microorganisms that attach to surfaces and allow them to survive in new and sometimes hostile environments. The ability of microbes to generate biofilms on indwelling and prosthetic medical devices has become a troublesome medical problem. C. neoformans biofilms allow for increased antifungal resistance. We are currently developing an in vivo model for C. neoformans biofilm formation. In addition, we are also investigating how antibodies may protect the host by preventing C. neoformans biofilm formation.
Phagosome extrusion
C. neoformans has the capacity to survive and replicate within the phagolysosome of macrophage cells. After multiplying, multiple fungal cells have been observed to be released, a process termed extrusion, from the immune cell and continue to survive. It has also been observed that this process of extrusion often involves a giant phagosome, suggesting that homotypic phagosome fusion occurs prior to extrusion. Our research focuses on the identification of genes that may be involved in phagosome fusion, and determining whether silencing these genes prevents subsequent extrusion. We are also interested in studying the possible role of annexins which function to bring cellular membranes in close proximity to each other to facilitate fusion in the process of extrusion.
Origin of virulence in pathogenic fungi
The mechanism of acquiring and maintaining virulence by C. neoformans is unknown. The evolution of virulence traits is being studied in three different systems. The interactions of C. neoformans with the free-living soil amoeba Acanthamoebae castellanii and the slime mold Dictyostelium discoideum are being characterized. These interactions help us understand the environmental survival strategy of C. neoformans and relate to the emergence of fungal virulence for humans. In particular, we are interested in the evolution of virulent traits in environmental microbes that do not require a host to complete their life cycle. We are working on the selective forces that drive heat tolerance acquisition (a necessary virulence factor for mammalian pathogens) within saprobic fungi in the Tremellales, the taxa that includes the human pathogenic cryptococci C. neoformans and C. gattii.
|
 |