Research
1. Prediction of metastatic risk based on tumor genomic and RNA sequencing data.
Alex Pearlman, Ph.D. (former student and postdoc) and I are working with Caris Life Sciences to replicate our previously reported pan-cancer metastasis profile score (panMPS) on their Caris Code AI platform and 484,000 patient clinico-genomics database. panMPS can be used to predict likelihood of metastasis from genomic and RNA sequencing data for a variety of cancers and to identify new therapeutic targets to treat aggressive cancers.
Table 1. Univariate logistic regression model of panMPS predicts progression to metastasis for cancers
| Cohort | MSK prostate cancer (n = 182, mPT = 25, iPT = 157) | Duke prostate cancer (n = 61, mPT = 37, iPT = 24) | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Odds Ratio | P | 95% CI | AUC | Odds Ratio | P | 95% CI | AUC |
| panMPS | 6.01 | 0.001 | 2.21 to 17.89 | 0.71 | 11.39 | 0.004 | 2.39 to 70.36 | 0.72 |
Table 2. Univariate logistic regression model of panMPS predicts progression to metastasis for cancers
| Cohort | Montefiore triple negative breast cancer (n = 41, mBC = 28, iBC = 13) | MSK lung adenocarcinoma (n = 33, mLA = 23, iLA = 10) | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Odds Ratio | P | 95% CI | AUC | Odds Ratio | P | 95% CI | AUC |
| panMPS | 44.74 | 0.02 | 2.91 to 1927.9 | 0.75 | 3.45×103 | 0.006 | 41.5 to 1.26×107 | 0.94 |
2. Integrative AI and single-cell spatial profiling to identify high-risk prostate cancer patients
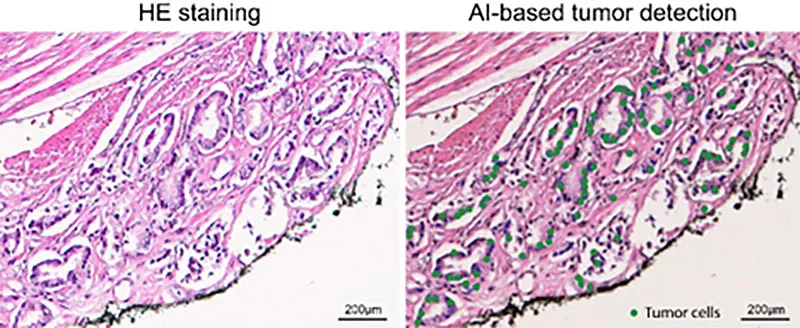
Shanye Yin (Assistant Professor of Pathology) and I are working together to use advanced spatial profiling and artificial intelligence to improve detection of residual cancer cells and identify patients at high risk of recurrence after surgery. The resulting tools (including application of AI to H&E sections) will enable more accurate and personalized treatment decisions, ultimately improving outcomes and reducing mortality for men with prostate cancer.
Fig. 1. AI-based identification of malignant cells within or close to the surgical margin. The AI classifier highlights tumor cells in green, while non-tumor cells remain unmarked.
3) Mechanisms of pathogenesis of human differences of sex development (DSDs).
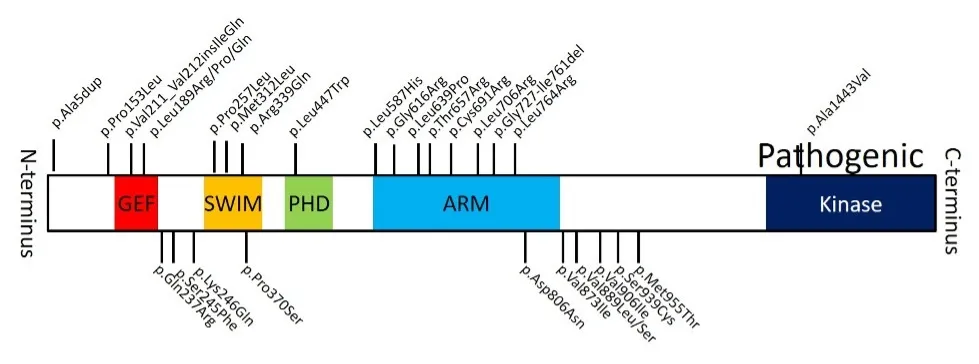
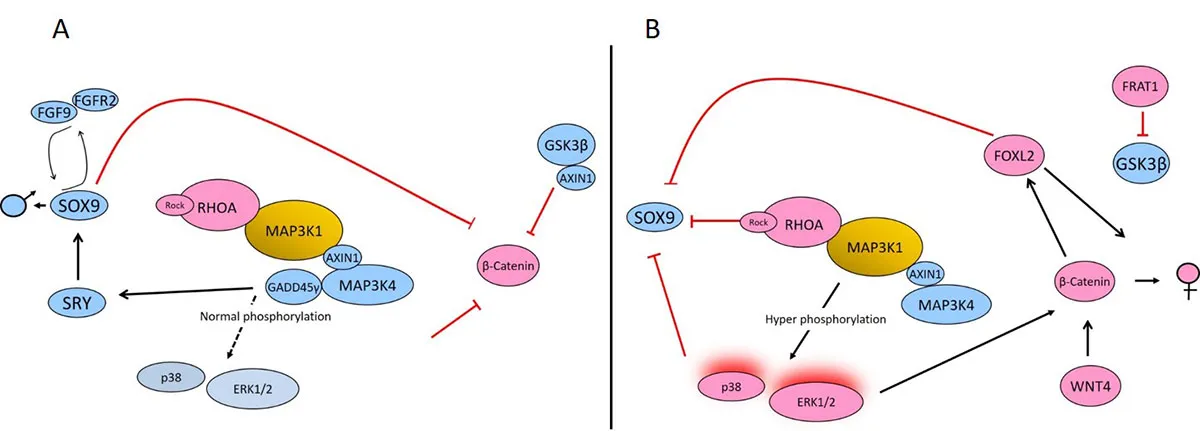
Previously, my laboratory showed that germline gain-of-function pathogenic variants are the most common cause of 46,XY DSD. These variants occur in or near known functional domains to increase phosphorylation of downstream targets, p38 and ERK1/2, and disrupt feedback and feedforward loops in the testis-determining pathway.
Fig. 2. Pathogenic variants in MAP3K1 (shown above the diagram) and benign variants (shown below the diagram)
Fig. 3. Role of MAP3K1, cofactors, and downstream targets in promoting gonadal determination.
Currently, I am working with collaborators from the University of Cape Town and Weill Cornell Medical College to perform long-read genomics sequencing to identify pathogenic variant(s) in a cohort of subjects with 46,XX ovotesticular DSD, the most common DSD among Black South Africans.
Reference: Pathogenic Variants in MAP3K1 Cause 46,XY Gonadal Dysgenesis: A Review.