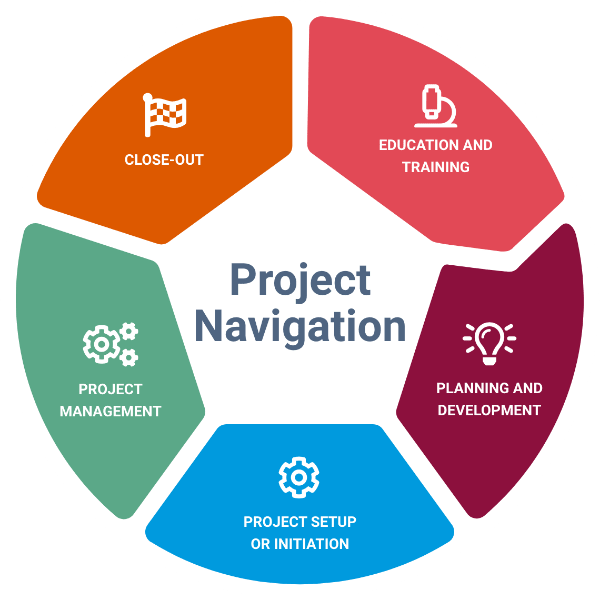
Overview
This interactive website provides a robust system for educating staff engaged in clinical and translational research, from novice to expert, on study procedures and regulatory requirements throughout the human subject research lifecycle. Included here, you will find relevant information, contacts, forms, templates, and other useful tools to organize your research.
According to 45 CFR 46, a human subject is "a living individual about whom an investigator (whether professional or student) conducting research obtain
- Information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or
- Uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens."
Refer to the NIH’s decision tool “am I doing human subjects research?” for further guidance.
Upcoming Events

Wednesday, March 18 from 12:00pm - 02:00pm
News & Highlights

The ICTR has promoted Jeff LaFleur, MA as the new Translational Core Director for the Biomarker Analytic Research Core and the Biorepository, both directed by Dr. Matthew Abramowitz. Jeff was previously the Translational Core Administrator and prior...

Matthew Abramowitz, MD, MS, has been named Director of the Montefiore Einstein Biomarker Analytic Research Core and Biorepository Core of the ICTR. Dr. Abramowitz is a longstanding user of the BARC/BioR services for supporting clinical & translational research...