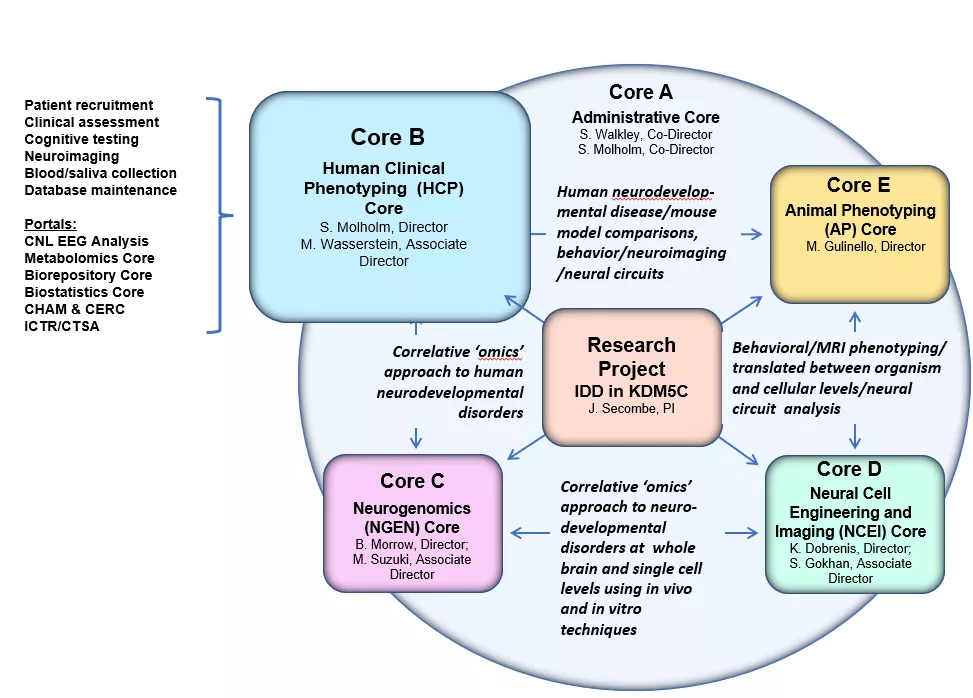
Human Clinical Phenotyping (HCP) Core
HCP Core Director:
Dr. Sophie Molholm
HCP Core Associate Director
Dr. Melissa Wasserstein
Objectives
The overarching mission of the Human Clinical Phenotyping Core (HCP) is to support innovative human-based IDD research at the Albert Einstein College of Medicine/Montefiore Medical Center. Through this effort we can better understand, effectively treat, and possibly prevent intellectual and developmental disabilities (IDDs) in children. The HCP Core serves as the bridge between basic researchers and clinical partners, facilitating human projects on a range of topics and populations by:
- Providing sophisticated phenotyping of participants for RFK-IDDRC investigators via clinical evaluations and diagnoses, all performed by highly qualified clinicians”
- Maintaining an extensive research-participant database (currently containing 2000+ children and adults) to service important IDD research endeavors
- Facilitating collaboration between the other RFK IDDRC Cores and the KDM5C Research Project by centralizing the data obtained from the Neurogenomics (NGEN) Core, the IDDRC Research Project, and the Gruss Magnetic Resonance Research Center for Imaging.
- Developing cutting-edge measures of IDD-relevant phenotypes, such as repetitive movement and multisensory processing
- Promoting research to and recruiting from our local Bronx community
Resources/Services
Recruitment:
We recruit participants for studies through several means including:
- the existing database of 2000+ participants;
- newspaper advertisements;
- Einstein and Montefiore clinical services;
- online list-serves, parent groups, local chapters of advocacy organizations, and special interest websites; and
- participation in community health fairs.
Phenotyping
Each child enrolled for participation in an investigator-initiated study is carefully phenotyped through a comprehensive set of basic tests. Investigators consult with our clinical psychologist and Core Director to determine the most appropriate battery of clinical and cognitive assessments and questionnaires for a given study question/population, taking into consideration both time constraints and available resources. The standard protocol includes neuropsychological tests of cognitive abilities and language function, sensory processing, adaptive functioning, and diagnostic tests as applicable. Additional measures are obtained through interviews with the parent/legal guardian, family pedigree charting to probe for familial occurrence of IDDs and neuropsychiatric disorders, taking photographs and video of the child, and basic vision and hearing screens. Further clinical assessments are administered as necessary for the specific project for which they are recruited/contacted. Our resources include EyeLink 1000 eye-trackers available for use in eye-tracking studies, for which the staff at the adjacent Cognitive Neurophysiology Laboratory has expertise in interfacing the system with Presentation (stimulus presentation and response acquisition software), EEG recording systems, and gaze-gated stimulation paradigms. We also collect saliva samples from willing participants and their families, process them for DNA, and then store them in the Einstein Biorepository. Thus, as new genetic pathways of disease are suggested in the literature, a substantial database of genetic materials that are allied with comprehensive phenotyping will be available to IDDRC investigators.
- Database: In support of an expansion of the HCP, we have developed a central database that is implemented with REDCap. The database incorporates different access levels and is compliant with HIPAA privacy rules. Specific populations may be queried for diagnostic features (e.g., autism, ADHD, high-risk psychosis, etc.) as well as several other variables that delimit the population of interest (e.g., age, sex, age of parents at birth, etc.). Additionally, we coordinate with the NGEN Core and the Gruss Magnetic Resonance Research Center for Imaging to index data collected from HCP participants. The HCP Core coordinates with investigators to maintain compliance with NIH data registries.
