Breast Cancer Research
Test Ties Chemotherapy to Possible Breast Cancer Spread
July 6, 2017—BRONX, NY—Using a test for predicting whether tumors will metastasize, or spread, a research team led by scientists at Einstein and Montefiore found that administering chemotherapy to mouse models of breast cancer-caused cellular changes associated with increased risk for metastasis. Similar results were observed for a small number of breast cancer patients who had previously undergone chemotherapy.

Maja H. Oktay, M.D., Ph.D.The study, published online July 5 in Science Translational Medicine, was led by Maja H. Oktay, M.D., Ph.D., professor of pathology and of anatomy and structural biology; George S. Karagiannis, D.V.M., Ph.D., postdoctoral fellow in the department of anatomy and structural biology; and John S. Condeelis, Ph.D., Judith & Burton P. Resnick Chair in Translational Research and professor and co-chair of anatomy and structural biology.
The experimental test for predicting whether tumors will spread was developed by Dr. Condeelis, who is also the co-director of the Gruss Lipper Biophotonics Center and its Integrated Imaging Program, and his Einstein colleagues. They had observed that breast cancer spreads to other parts of the body when three specific cells are in direct contact: an endothelial cell (a type of cell that lines blood vessels), a Tie2-Hi perivascular macrophage (a type of immune cell involved in blood-vessel growth) and a tumor cell that produces high levels of Mena, a protein that enhances a cancer cell’s ability to invade blood vessels and spread. Where these three cells come in contact is where tumor cells can enter blood vessels—a site called a tumor microenvironment of metastasis, or TMEM. Tumors with high numbers of TMEM sites (i.e., they have a high TMEM “score”) were more likely to metastasize compared with tumors with lower TMEM scores.

John S. Condeelis, Ph.D.In the Science Translational Medicine study, TMEM testing was carried out on breast tissue of three different breast-cancer mouse models that had been treated with the chemotherapy agent paclitaxel. For all three animal models that had undergone chemotherapy, TMEM activity was increased in the live tumors. In addition, TMEM scores for mice that had undergone chemotherapy were two-to-three fold higher compared with TMEM scores of mice not receiving chemotherapy.Using a different chemotherapy regimen—doxorubicin plus cyclophosphamide—led to similar findings. TMEM testing on biopsy specimens from residual breast cancers of 20 women previously treated with paclitaxel followed by doxorubicin plus cyclophosphamide showed that most patients had an increase in TMEM scores after chemotherapy, and five patients experienced more than a five-fold increase in TMEM score compared with scores before chemotherapy.
Watch Dr. Condeelis describe his research
The study also found that the drug rebastinib, a Tie2 inhibitor that blocks TMEM function, when administered to breast-cancer mouse models treated with paclitaxel, blocked both the blood vessel permeability and cancer-cell spread associated with TMEM.
The study provides insights into the mechanisms of chemotherapy induced cancer cell dissemination, potential targeted therapies to block chemotherapy-induced cancer cell dissemination and potential predictive markers to determine who may or may not benefit from pre-operative chemotherapy.
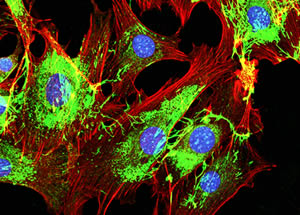
Metastatic cancer cells spreading on the surrounding tissueThe study is titled “Neoadjuvant Chemotherapy Induces Breast Cancer Metastasis through a TMEM-Mediated Mechanism.” In addition to Drs. Oktay, Karagiannis, and Condeelis, other authors are Jessica M. Pastoriza, M.D., Yarong Wang, M.S., Allison S. Harney, Ph.D., David Entenberg, M.Sc., Jeanine Pignatelli, Ph.D., Ved P. Sharma, Ph.D., Emily A. Xue, Joan G. Jones, M.D., Jesus Anampa Mesias, M.D., Thomas E. Rohan, M.B.B.S., Ph.D., and Joseph A. Sparano, M.D., all at Einstein and Montefiore; and Esther Cheng and Timothy M. D’Alfonso, M.D., at Weill Cornell Medicine.
The study was funded by grants from the National Institutes of Health (CA100324; CA150344; CA200561; S10OD019961), and Albert Einstein College of Medicine. Dr. Condeelis and Oktay are inventors on a patent application submitted by Einstein that covers methods of detecting and reducing chemotherapy-induced pro-metastatic changes in breast tumors. All other authors declare that they have no competing interests.
Other Top Stories
9/11 World Trade Center Exposure Linked to Heart Disease Among NYC Firefighters
On Becoming a Physician: New Einstein Students Receive White Coats and Stethoscopes
Novel Therapy for Acute Migraine Shows Promise in Phase 3 Clinical Trial
First Complete Wiring Diagram of an Animal's Nervous System
Multimillion Dollar NIH Grant to Help Reduce Opioid Use & Get Care to People Who Need It
NIH Grant Funds $23 Million Study of Diseases Affecting People Living with HIV
New TAILORx Data Guides Adjuvant Therapy in Younger Breast Cancer Patients
Einstein Celebrates Its 61st Commencement
Bolstering Biopsies: Testing Patients' Individual Cells to Guide Treatment



Tablet Blog