FULL STORY
Researchers Find Clue In Missing Protein Related to Common Form of Inherited Mental Retardation
June 13, 2008 - (BRONX, NY) - Researchers at Emory University School of Medicine and Albert Einstein College of Medicine of Yeshiva University have helped to discover important new information about the cause of Fragile X Syndrome, the most common form of inherited mental retardation. The study appears in the June 10 issue of Developmental Cell, and was led by Gary J. Bassell, professor of cell biology and of neurology at Emory and Robert H. Singer, Ph.D., professor and co-chair of anatomy and structural biology at Einstein.
 Fragile X syndrome is caused by the absence of a protein known as FMRP (fragile x mental retardation protein). FMRP is necessary for brain signaling at the synapse, the site where two neurons communicate via chemical and electrical systems. This signaling is essential for normal brain development, learning and memory.
Fragile X syndrome is caused by the absence of a protein known as FMRP (fragile x mental retardation protein). FMRP is necessary for brain signaling at the synapse, the site where two neurons communicate via chemical and electrical systems. This signaling is essential for normal brain development, learning and memory.
The researchers found how the lack of FMRP in Fragile X Syndrome interferes with this vitally important signaling process. Their results may lead to the development of new treatments for Fragile X Syndrome, which also contributes significantly to autism spectrum disorders and epilepsy.
FMRP—the protein that causes Fragile X Syndrome when absent—is known to be an mRNA (messenger RNA) binding protein. mRNA's are the molecules that convey the DNA sequences of genes from the cell nucleus out to the cytoplasm where the mRNA message is transcribed into proteins. By regulating mRNA molecules, mRNA binding proteins crucially influence how proteins are synthesized from mRNAs.
The precise functions for FMRP have been unclear, but scientists recently have learned that FMRP can bind to and regulate several mRNAs that are present at synapses in the brain. Each mRNA molecule can be translated many times at the synapse, producing many copies of the encoded protein and providing an efficient way for a neuron to supply its synapse with essential proteins needed for communication.
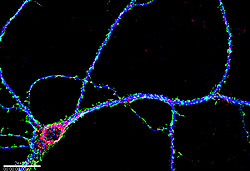 Until now, it wasn't clear how FMRP and its bound mRNAs are delivered to the tentacle-like projections of neurons—their axons and dendrites--and then on to the synapses at their outer extremities.
Until now, it wasn't clear how FMRP and its bound mRNAs are delivered to the tentacle-like projections of neurons—their axons and dendrites--and then on to the synapses at their outer extremities.
"A major question in neuroscience is how mRNAs that code for proteins crucial for neuron-to-neuron communication can be precisely transported over long distances to where they are needed--and then be regulated at exactly the right moment to permit communication between neurons, ultimately leading to learning and memory," said Dr. Singer, who also is professor of neuroscience at Einstein.
"FMRP is a protein that can target messenger RNAs destined for the farthest reaches of the cell and deliver them at the right place and time for synaptic signaling to occur," added Dr. Bassell.
 The researchers and their colleagues have developed high resolution microscopic imaging tools to visualize FMRP in live neurons, allowing them to track the movements of FMRP and its associated mRNA molecules along dendrites, using cultured neurons isolated from the hippocampus of mouse embryos. They discovered that FMRP binds to a molecular motor, which allows it to carry its bound mRNAs in the form of particles out into the dendrites.
The researchers and their colleagues have developed high resolution microscopic imaging tools to visualize FMRP in live neurons, allowing them to track the movements of FMRP and its associated mRNA molecules along dendrites, using cultured neurons isolated from the hippocampus of mouse embryos. They discovered that FMRP binds to a molecular motor, which allows it to carry its bound mRNAs in the form of particles out into the dendrites.
Using a mouse model of Fragile X Syndrome, the investigators saw that mRNAs were not motored into dendrites in response to synaptic signaling. The result: a lack of the local protein synthesis at synapses that is needed to sustain the signaling between nerve cells. In essence, Fragile X Syndrome occurs because neurons have lost the ability to send protein-coding messages from their nuclei to their synapses.
The researchers also were able to identify the select group of mRNAs that, under normal circumstances, the neuron ships into dendrites via FMRP. Knowing which molecules within the FMRP pathway function at synapses should facilitate the development of new treatment strategies and drug interventions for Fragile X Syndrome.
In addition to senior authors Drs. Bassell and Singer, the lead author on the paper was Jason B. Dictenberg of Hunter College, City University of New York and Albert Einstein College of Medicine. Other researchers were Sharon A. Swanger, Emory University Department of Cell Biology and Laura N. Antar, Albert Einstein College of Medicine.
The National Institutes of Health and the Fragile X Research Foundation funded the research.
###
Other Top Stories
9/11 World Trade Center Exposure Linked to Heart Disease Among NYC Firefighters
On Becoming a Physician: New Einstein Students Receive White Coats and Stethoscopes
Novel Therapy for Acute Migraine Shows Promise in Phase 3 Clinical Trial
First Complete Wiring Diagram of an Animal's Nervous System
Multimillion Dollar NIH Grant to Help Reduce Opioid Use & Get Care to People Who Need It
NIH Grant Funds $23 Million Study of Diseases Affecting People Living with HIV
New TAILORx Data Guides Adjuvant Therapy in Younger Breast Cancer Patients
Einstein Celebrates Its 61st Commencement
Bolstering Biopsies: Testing Patients' Individual Cells to Guide Treatment



Tablet Blog