FULL STORY
Einstein Cancer Center Receives $10 Million Grant for Pioneering Cancer Research
November 06, 2008 — Bronx, NY — Researchers in the Cancer Center of Albert Einstein College of Medicine of Yeshiva University have received a five-year, $10-million grant renewal from the National Cancer Institute (NCI) to study how cancer cells spread, or metastasize. Understanding this process is crucial, since most of the 553,000 U.S. cancer deaths each year are caused by complications from the spread of cancer to distant tissues and organs, rather than from the primary tumor itself.

Members from the laboratories of Drs. John Condeelis, Jeffrey Segall
and Dianne Cox at the Albert Einstein Cancer CenterIn previous research funded by NCI (part of the National Institutes of Health), Einstein researchers demonstrated that immune system scavenger cells called macrophages play an important role in how cancer is spread. The research team determined that these macrophages enhance the ability of tumor cells to move and to invade tissues.
Here's how the process works: macrophages cluster along blood vessels that feed a tumor. These clusters emit chemical signals that make it easier for tumor cells to enter the blood vessels, a process called intravasation. Once in the bloodstream, tumor cells are able to travel to sites throughout the body.
"The contribution of intravasation to metastasis has not been clear, and this has led many researchers to look elsewhere for potential therapeutic interventions," says the principal investigator, Dr. John Condeelis, co-chair and professor of anatomy and structural biology. "Our findings have completely turned this around."
The discoveries were made possible by an imaging technology called intravital multiphoton microscopy. Developed by Dr. Condeelis and colleagues, this technology allows researchers to capture high-resolution, three-dimensional moving images revealing the behavior of individual cells deep within living tissues.
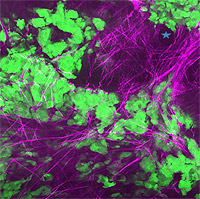
Metastasis of a breast tumor
in a live mouse. Green tumor
cells are seen migrating on
purple collagen fibers toward a
blood vessel.In the program's current phase, Einstein cancer researchers aim to identify the subtypes of macrophages involved in metastasis and to clarify how each of these cell types affect the development of blood vessels (angiogenesis), the movement of cancer cells into the bloodstream (intravasation), and the movement of cancer cells from blood vessels into organs (extravasation).
This large program project grant is part of activities in the Tumor Microenvironment and Metastasis Program, one of seven interdisciplinary programs of the Albert Einstein Cancer Center. Investigators in the five projects of this program project grant include Jeffrey W. Pollard, Ph.D. (Tumor Progression and Metastasis), E. Richard Stanley, Ph.D. and Dianne Cox, Ph.D. (CSF-1R Signaling Pathways Regulating Macrophage Chemotaxis and Angiogenic Factor Release), Jeffrey E. Segall, Ph.D. (ErbB1 and ErbB2 Roles in Invasion and Intravasation), and Jonathan Backer, M.D. and Anne Bresnick, Ph.D. (PI 3-Kinase and Metastasis). Dr. Condeelis leads the fifth project focusing on translation of findings from this laboratory research to the treatment of patients with breast cancer. Dr. Condeelis is responsible for the overall scientific design and direction of the program project grant.
The researchers hope to define various factors and biochemical pathways that regulate the spread of cancer cells at the molecular level. In addition, they will evaluate biomarkers — identified in earlier studies — for their ability to predict outcomes in human breast cancer.
The research has important implications for therapy. According to Dr. Condeelis, treatments that prolong life and improve quality of life of cancer patients will probably depend on a combination of therapies; those that minimize the growth of existing tumors and others that limit their spread to new sites. To develop the latter, researchers will need a detailed understanding of the basic steps of metastasis — the ultimate goal of this research.
###
Other Top Stories
9/11 World Trade Center Exposure Linked to Heart Disease Among NYC Firefighters
On Becoming a Physician: New Einstein Students Receive White Coats and Stethoscopes
Novel Therapy for Acute Migraine Shows Promise in Phase 3 Clinical Trial
First Complete Wiring Diagram of an Animal's Nervous System
Multimillion Dollar NIH Grant to Help Reduce Opioid Use & Get Care to People Who Need It
NIH Grant Funds $23 Million Study of Diseases Affecting People Living with HIV
New TAILORx Data Guides Adjuvant Therapy in Younger Breast Cancer Patients
Einstein Celebrates Its 61st Commencement
Bolstering Biopsies: Testing Patients' Individual Cells to Guide Treatment



Tablet Blog