
Francisco Lazaro-Dieguez, Ph.D.
- Instructor, Department of Developmental & Molecular Biology
Area of research
- Cell biology; protein trafficking and cell polarity development in hepatocytes.
Phone
Location
- Albert Einstein College of Medicine Jack and Pearl Resnick Campus 1300 Morris Park Avenue Chanin Building 517 Bronx, NY 10461
Research Profiles
Professional Interests
The most common cells of the liver (hepatocytes) are polarized epithelial cells, with their apical/canalicular and basolateral domains separated by tight junctions, that form a barrier between the sinusoids and the bile canaliculi ‒intercellular spaces formed by the apical membranes of adjacent hepatocytes‒. Hepatocyte polarization is crucial for the liver function. Key elements in hepatocyte polarization include the extracellular matrix, cell-cell junctions, and the intracellular protein trafficking machinery.
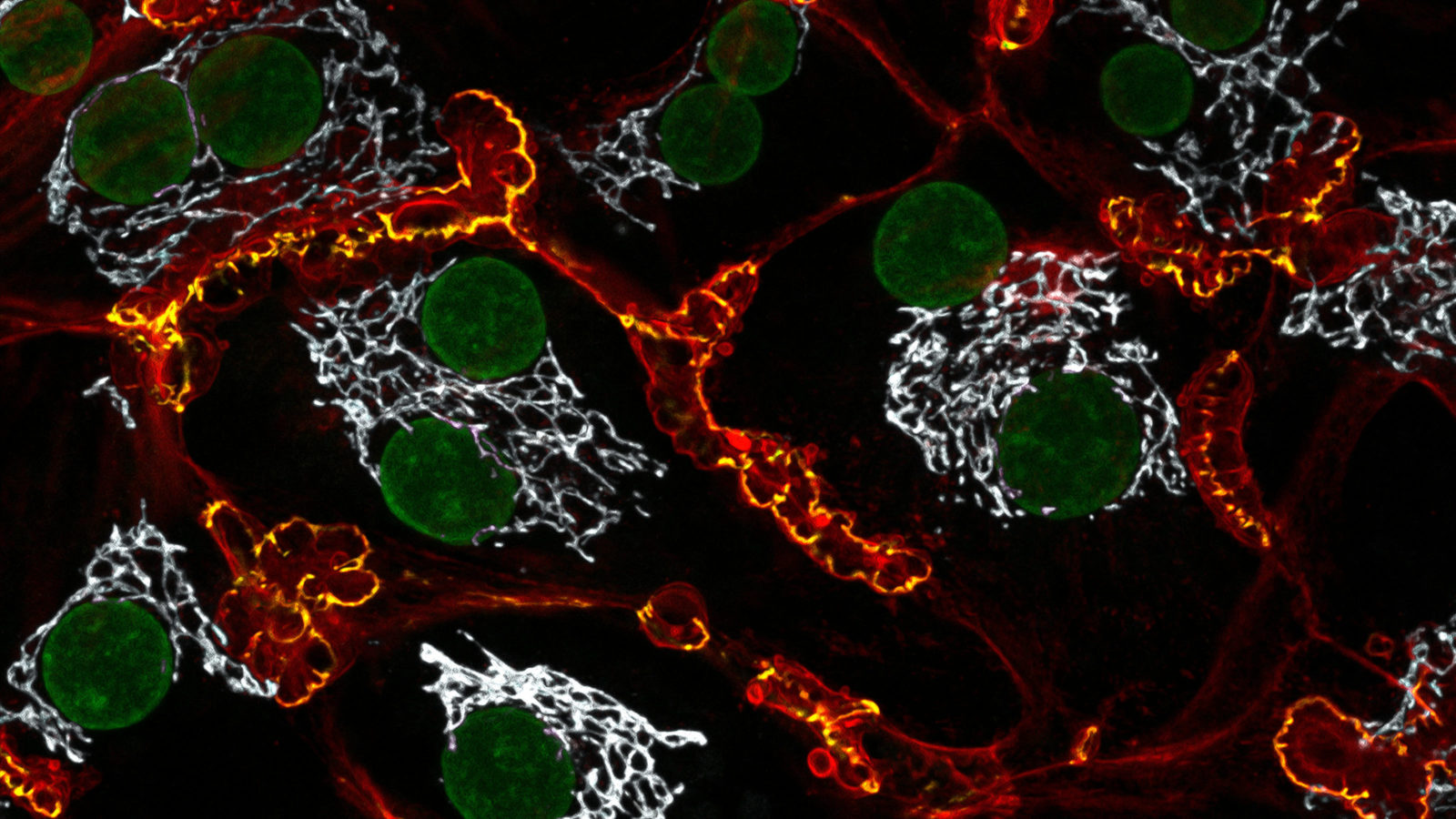 Primary rat hepatocytes showing actin filaments, tight junctions, TGN, and nuclei.
Primary rat hepatocytes showing actin filaments, tight junctions, TGN, and nuclei.
Disruption of the bile canaliculi architecture and hepatocytic polarity are hallmarks of liver disease, including cholestasis and cancer. The understanding of the molecular and cellular mechanisms governing hepatocyte polarity therefore is essential to both liver cell biology and the pathogenesis of liver diseases. We reported that Rho activation prevents hepatocytic polarity and the exit of apical and basolateral proteins from the trans-Golgi network (TGN) in common carries (Traffic 21, 364-74, 2020).
 Exit of apical and basolateral cargo from the TGN.
Exit of apical and basolateral cargo from the TGN.
My current research at Dr. Muesch lab focuses on the study of the molecular mechanisms that govern the formation and manteinance of the bile canaliculi.
Selected Publications
Lazaro-Dieguez, F.*, Musch, A. Live-cell Imaging of Biosynthetic Protein Transport in Hepatocytes. Methods Mol Biol. 2544, 145-57, 2022. PMID: 36125716
Lazaro-Dieguez, F.*, Musch, A.* Low Rho Activity in Hepatocytes Prevents Apical from Basolateral Cargo Separation during trans‐Golgi Network to Surface Transport. Traffic 21, 364-74, 2020. PMID: 32124512 (Recommended by Faculty Opinions)
Lazaro-Dieguez, F.*, Musch, A.* Cell‒Cell Adhesion Accounts for the Different Orientation of Columnar and Hepatocytic Cell Divisions. J. Cell Biol. 216, 3847-59, 2017. PMID: 28887437
Lazaro-Dieguez, F., Ispolatov I., Musch, A. Cell Shape Impacts on the Positioning of the Mitotic Spindle with Respect to the Substratum. Mol. Biol.Cell. 26, 1286-95, 2015. PMID: 25657320 (Highlighted article,+ Cover art)
Lazaro-Dieguez, F., Cohen, D., Fernandez, D., Hodgson, L., van IJzendoorn, S.C.D., Musch, A. Par1b Links Lumen Polarity with LGN‒NuMA Positioning for Distinct Epithelial Cell Division Phenotypes. J. Cell Biol. 203, 251-64, 2013. PMID: 24165937
Cohen, D., Fernandez, D., Lazaro-Dieguez, F., Musch, A. The Serine/Threonine Kinase Par1b Regulates Epithelial Lumen Polarity via IRSp53-Mediated Cell-ECM Signaling. J. Cell Biol. 192, 525-40, 2011. PMID: 21282462
del Toro, D., Alberch, J., Lazaro-Dieguez, F., Martin-Ibanez, R., Xifro, X., Egea, G., Canals J.M. Mutant Huntingtin Impairs post-Golgi Trafficking to Lysosomes by Delocalizing Optineurin/Rab8 Complex from the Golgi Apparatus. Mol. Biol. Cell. 20, 1478-92, 2009. PMID: 19144827
Lazaro-Dieguez, F., Aguado, C., Mato, E., Sanchez-Ruiz, Y., Esteban, I., Alberch, J., Knecht, E., Egea, G. Dynamics of an F-actin Aggresome Generated by the Actin-Stabilizing Toxin Jasplakinolide. J. Cell Sci. 121, 1415-25, 2008. PMID: 18398002 (+ Covert art)
Lazaro-Dieguez, F., Jimenez, N., Barth, H., Koster, A.J., Renau-Piqueras, J., Llopis, J., Burger, K.N., Egea, G. Actin Filaments Are Involved in the Maintenance of Golgi Cisternae Morphology and Intra-Golgi pH. Cell Motil. Cytoskeleton. 63, 778-91, 2006. PMID: 16960891 (+ Cover art)
Egea, G., Lazaro-Dieguez, F., Vilella, M. Actin Dynamics at the Golgi Complex in Mammalian Cells. Curr. Opin. Cell Biol. 18, 168-78, 2006. PMID: 16488588
* Corresponding author





