Research
The aggregation of membrane receptors during cell adhesion initiates the elaborate networks of signaling pathways. The complexities of the networks originate from the spatial-temporal interactions of their numerous cellular components.
By integrating computational analysis with experimental measurements, our lab is focusing on developing a multi-scale modeling framework to understand the molecular mechanisms of protein interactions underlying the physics of cell adhesion, as well as their biological significance.
Methodologies
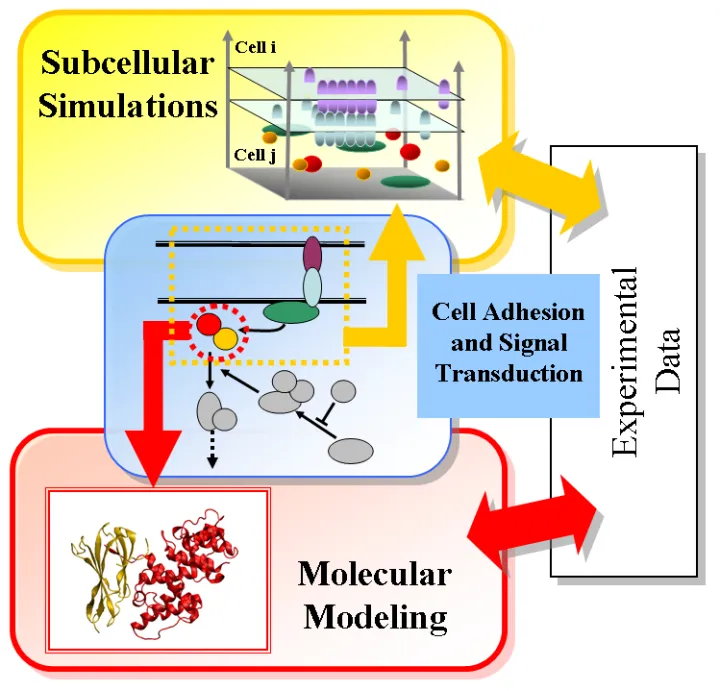
The development of a multi-scale modeling framework could lead to an integrative understanding of how extracellular signals regulate cell adhesion and downstream signaling pathways in various biological systems. By designing different simulation scenarios on molecular level, sub-cellular level, systems level and multi-cellular level, the framework could serve as a guide to reveal the molecular mechanism of specific disease-related problems.
Applications
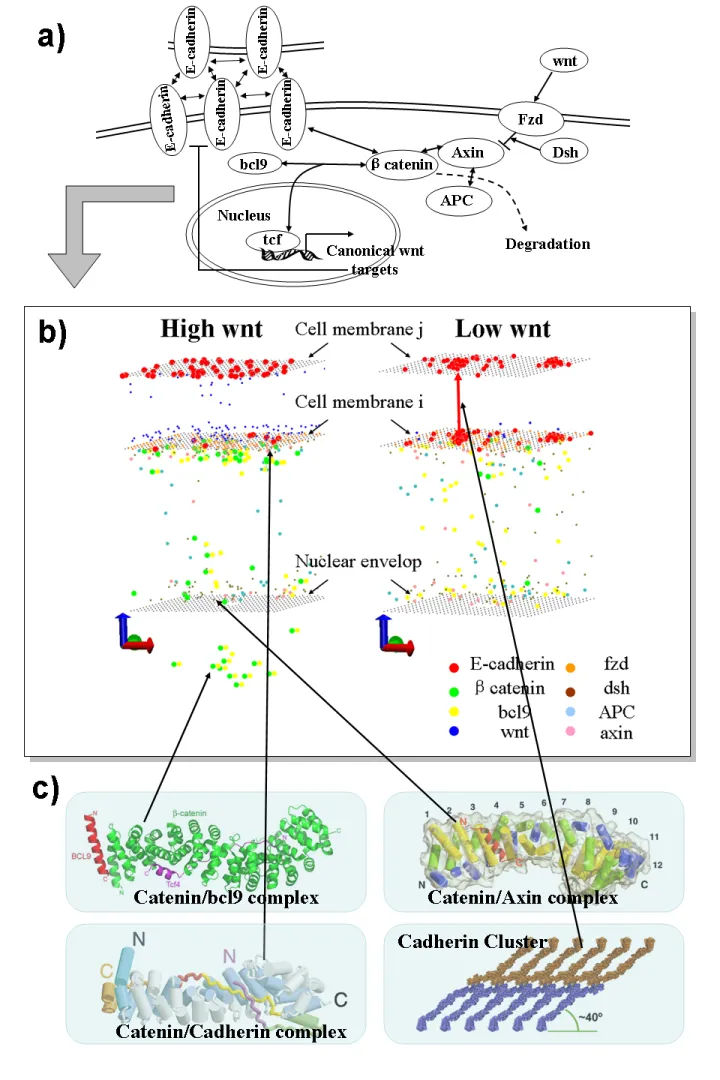
Cadherin/Wnt Signaling
The epithelial-mesenchymal transition (EMT), characterized by repression of cell adhesion, is the hallmark of both normal embryonic development and cancer metastasis. Wnt is one of the most important signaling pathways triggering EMT. The key players in Wnt signaling is β-catenin, which is involved in both intercellular adhesion and gene regulation. The binding of β-catenin to the cytoplasmic domain of E-cadherin results in the stabilization of adherens junctions. On the other hand, its association with the T-cell factor/lymphoid enhancer factor (TCF/LEF) DNA binding proteins changes the transcription of target genes, initializing the canonical Wnt pathway. The fate of β-catenin in adhesion and signaling is further regulated by Wnt activation and its downstream phosphorylations. As the functions of β-catenin have been studied separately in cadherin-based junction formation and in Wnt signaling pathway, relatively little has been done to connect these two systems. Our goal for this project is to quantitatively interrogate the interplay between cadherin-mediated junction formation and canonical Wnt signaling pathway by asking the direct question: How can competition of β-catenin between these two systems serve to integrate cell adhesion with gene expression?
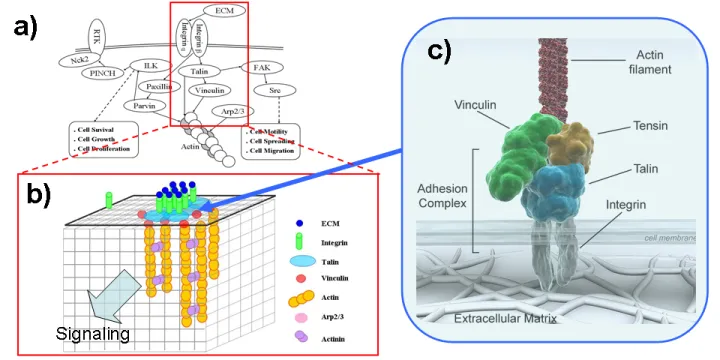
Integrin Signaling
During cell migration, large macromolecular assemblies form at focal adhesions to transmit mechanical force and regulatory signals across cell membranes. Integrins serve as the mechanical linkages to the extracellular matrix (ECM). Their clustering based on ligand binding provides a biochemical signaling hub to direct numerous signaling and adapter proteins such as talin and focal adhesion kinase (FAK). We study the molecular mechanism of integrin clustering and its impact on mechano-chemical coupling by multi-scale modeling. Our studies can be directly compared with cellular imaging experiments, and will give insights into the dynamic coupling of integrin clustering with downstream signaling events, for instance, the recruitment of FAK.
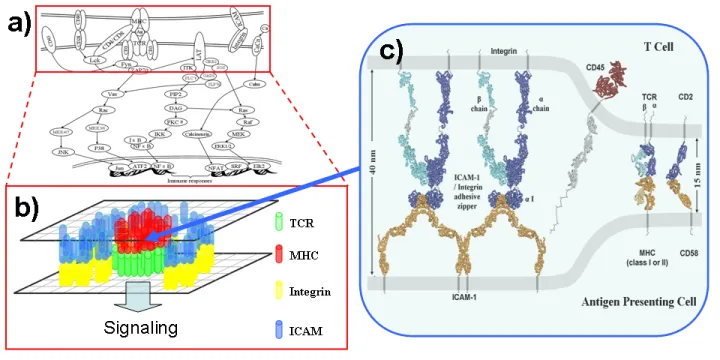
T-cell Signaling
T cells play a pivotal role in cell-mediated immunity. The spatiotemporal patterning between T-cells and antigen-presenting cells (APCs) leads to the maturation of the immunological synapse (IS). This process is highly correlated to T-cell activation. Although size of membrane receptors was suggested to drive synaptic patterning, detailed structural information has not been used to study such sub-cellular process. Combining knowledge from molecular and cellular levels, we are using multi-scale studies to understand why specific patterns can be formed on T cell surfaces and how they are related to the intracellular signaling.
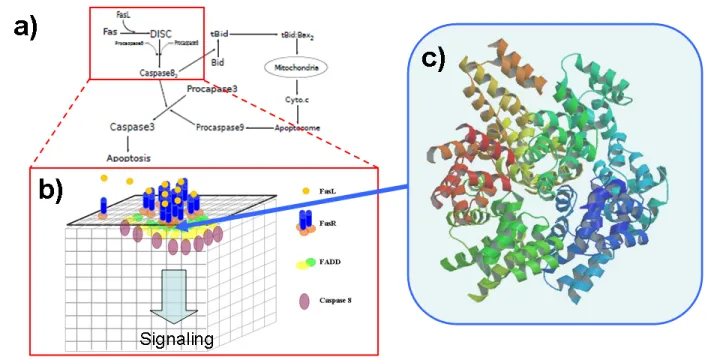
Death Domain Signaling
Death domains provide the platform to trigger the clustering of membrane receptors and initiate the recruitment of downstream caspases to induce cell apoptosis and immunological response, via formation of death-inducing signaling complexes (DISC). Recent crystal structures bring various pieces of evidence for the hierarchical assembly of death domains. However, the dynamic way they form complexes and their functional relationship to membrane receptor clustering and downstream signaling processes still remain unclear. Our primary goal in this project is to understand how receptor clustering triggers the formation of DISC.
