Metastasis, the spread of cancer cells from the primary tumor to other organs and systems of the body, causes 90 percent of deaths from cancer. What drives metastasis at the cellular and molecular levels? How effective is systemic therapy in halting the dissemination and proliferation of cancer cells? A coalition of clinicians and scientists from five top New York City medical research institutions, spearheaded by Montefiore-Einstein, is working to find answers.
Conceived by Maja H. Oktay, MD, PhD, a professor of pathology in the L.G. Koss Division of Cytology at Montefiore and of anatomy & structural biology at Albert Einstein College of Medicine, the New York Pathology Oncology Group (NYPOG) was launched in 2017. NYPOG member institutions include Montefiore-Einstein, NYU Langone, Mount Sinai Hospital, Memorial Sloan Kettering Cancer Center, and Weill Cornell Medical Center.
Dr. Oktay, the founding director of the NYPOG, discusses the NYPOG and its work; her career path as a pathologist and cancer cell biologist; and her interest in the tumor microenvironment, the perpetrator in metastatic breast cancer.
How did the NY Pathology Oncology Group come about?
In 2008, I started collaborating with Dr. Joan Jones and Dr. John Condeelis at Einstein, on the development of markers of cancer cell dissemination. As time went by, I became increasingly involved in studying the cellular and molecular aspects of pro-metastatic tumor microenvironments and the mechanisms of cancer cell intravasation through Tumor Microenvironments of Metastasis (TMEM) doorways—transient openings on blood vessels that lead to dissemination of cancer cells to distant organs.
As my efforts to secure research funding become more successful, I realized that we needed to develop multi-institutional collaborations, not only to increase the quantity and diversity of patient tissue samples, but also to generate a team of individuals with the expertise needed to address the problem of cancer cell dissemination from various angles.
What are the mission and goals of the NYPOG?
The NYPOG was formed to promote collaboration among physicians and scientists who are interested in pro-metastatic tumor microenvironments and the effect of systemic therapy on cancer cell dissemination and metastatic seeding. Our shared mission is to translate scientific discoveries into patient care, facilitated by collaborations and resources within the NCI-designated cancer centers in our four NYPOG member institutions.
While metastasis is a major cause of death from cancer, the ability of clinicians to predict which tumors will metastasize, and to treat potential re-dissemination of cancer cells from already formed metastatic foci, has been limited. Better understanding of the mechanisms of metastasis will advance our ability to prevent, treat and cure the metastatic disease.
Is NYPOG the only group of its kind focusing on metastasis?
I’m not aware of another group with a similar mission and vision in our geographic area. Of course, there are a few large international professional societies focused on studying cancer cell dissemination, such as The Metastasis Research Society (MRS). The mission of the MRS is to support progressive research on processes fundamental to metastasis, so it is much more general than the mission of the NYPOG. The MRS, like the NYPOG, encourages exchange of information between basic researchers, clinicians, industry and even patients. However, the NYPOG not only encourages the exchange of information among different fields, its work is literarily based and depends on multi-disciplinary collaborations. Another aspect of the NYPOG is that it’s specifically focused on discovering the potential differences in the process of cancer cell dissemination and the response to cancer treatment among patients of different racial and ethnic backgrounds. In this aspect the NYPOG is unique.
Who are the NYPOG members?
Our members represent a broad array of subspecialties: pathology, oncology, surgery, epidemiology, cancer biology, optical physics, engineering and biophysics. Some are individuals we collaborated with back in 2008. Many of the collaborations are new and have developed over the years. We now expect that our Einstein radiology team (Drs. Craig Branch, Laura Hodges and Tim Doung) will join NYPOG as well.
Do NYPOG members meet regularly?
Yes. In fact, just last week (early December, 2020), we held our quarterly NYOPOG meeting over Zoom. Before COVID, we would rotate the locations of our quarterly meetings among all participating institutions. This was a lot of fun because we made sure to have extra time for informal discussions and exchange of ideas, during the break between the two formal sessions. In addition, our working groups meet monthly by Zoom. That was in place even before COVID.
What are some of the benefits of collaborating with these other institutions?
Collectively, we serve a very diverse, urban population. The collaboration allows us to evaluate large multiracial patient cohorts and their samples. Also, we have access to resources and expertise that might not be available at each of our respective institutions.
Which laboratories from Einstein-Montefiore are involved?
We’re fortunate to have incredible resources here at Einstein.
For example:
- The Gruss Magnetic Resonance Research Center
- The Gruss Lipper Center for Biophotonics
- The Translational Pathology Satellite Core Lab
Can you give an example of a current study involving investigators from different NYPOG member institutions?
Sure! Currently, Einstein, Cornell, MSKCC and NYU are working together to evaluate racial disparity in the density of TMEM doorways in residual breast cancer in patients who received pre-operative chemotherapy. In addition, we’re evaluating the association of TMEM doorway density with the occurrence of metastasis in these patients. The preliminary data from patients at Einstein and Cornell showing that high TMEM doorway density is associated with worse distant-recurrence survival were presented at the San Antonio Breast Cancer Symposium in 2018. We’re also evaluating the use of TMEM doorway density and several other markers of pro-metastatic cancer cell phenotype in patients treated with post-operative chemotherapy. And we just finalized a manuscript showing that combining TMEM doorway density with the density of cancer cells that can use these doorways has significantly stronger prognostic power than either marker alone.
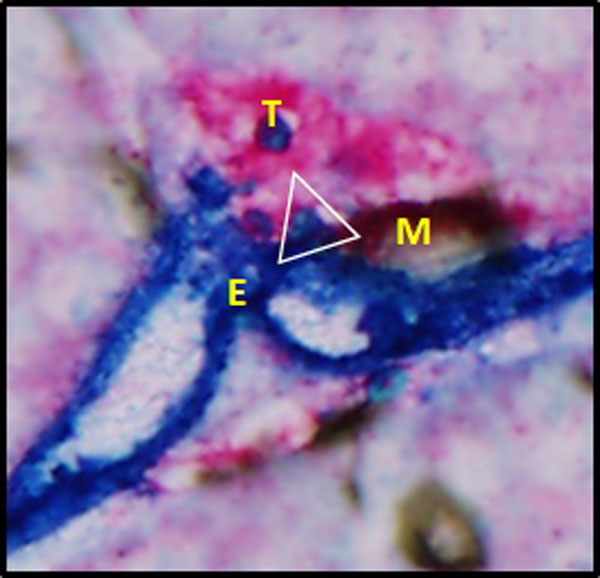
I notice that the NYPOG is interested in the use of Artificial Intelligence (AI) as a tool for predicting metastasis. Can you talk more about that?
NYPOG is using image analysis paired with specific algorithms to determine features in the tumor microenvironment associated with cancer cell dissemination and disease outcome. We haven’t discussed using AI yet, however we may do so sometime in the future. Since metastasis is such a complex process, AI may be extremely helpful.
Are you aware of the AI work of Dr. Kenji Ikemura, a third-year pathology resident at Montefiore?
Yes, this is fascinating work! Dr. Ikemura is an exceptionally bright and talented physician. Pairing medicine and engineering is something our group depends on as well. For example, our engineers, Dr. David Entenberg and Dr. Xianjun Ye (a postdoctoral research fellow in Dr. Condeelis’s lab),,have developed algorithms for automated analysis of TMEM doorway density. They’ve also developed algorithms that combine several aspects of the pro-metastatic tumor microenvironment into one more-powerful predictor of metastatic outcome. Isabelle Oktay, a summer intern majoring in computer science at New York University, developed cell-segmentation algorithms that were crucial for our image analysis. As the research community tackles increasingly complex problems, it will inevitably require expertise in both engineering and computer science. So, I completely agree with Dr. Ikemura, that progress in cancer research will increasingly depend on the involvement of individuals skilled in those two disciplines.

Your background in cell biology and cytopathology uniquely positions you for translational research. As a pathologist you bring a clinical perspective that enables you to be a bridge between basic science and the clinic.
What made you decide to pursue training in pathology?
When I was 16 years old, my mother was diagnosed with breast cancer. This was an extremely stressful experience; it made me decide to dedicate my life to studying cancer and discovering a cure for it. As a third-year medical student I got involved in cancer research. I then started seesawing between basic science and clinical work for a long time; I did my masters, and then my PhD. During my post-doctoral fellowship training at Memorial Sloan Kettering Cancer Center, I realized that I really wanted to study human disease and that pathology, a field that had extremely excited me since medical school, was the way to go. So, I got accepted into the anatomical pathology residency at Yale, which only solidified my desire to pursue a career in academic medicine. It was not until I completed my fellowship in cytopathology here at Einstein, and became an attending, that I realized that doing what I had wanted for so long was possible. Thanks to the support I received from our chairman, Dr. Michael Prystowsky; Dr. Mark Suhrland, the director of cytopathology; and Dr. Condeelis, the co-chair of anatomy and structural biology, my desire to become a physician-scientist materialized.
What skills in the pathologist’s toolbox have been especially valuable in your research?
It’s hard to pinpoint one particular skill from the pathologist’s toolbox that is particularly helpful in research. I would say that the whole pathology training gives one the unique understanding of tissues in the human body and how they’re altered with the disease such as cancer. It is really priceless for pursuing translational research in the cancer field. The training in fine needle aspiration (FNA) biopsy, part of the cytopathology fellowship curriculum, is definitely a bonus that enabled me to connect all the dots: the patient to the tumor, to the feeling of pulling cancer cells from the tumor, to the sensation of how cancer cells spread on the slide, to the appearance of the cancer cells on the slide, to the final diagnosis. Pathology and cytopathology truly marry the art and science of medicine.
What are some of the projects you’re working on now?
I’m working on several projects which all, from different perspectives, address the same problem: how the tumor microenvironment affects cancer cell dissemination, disease progression and patient survival. Our goal is to link the observations from the large amount of patient data, and the analyses of tissue samples from patients, with more mechanistic studies using pre-clinical mouse models and cell biology approaches. For example, our analysis of data from more than 10,000 patients showed a difference in breast cancer distant recurrence in relationship to race. The analyses of patient breast cancer tissue samples indicated that these differences may stem from the composition of the tumor microenvironment. In pre-clinical mouse models, we’re now pinpointing the specific cell-cell interactions within the tumor microenvironment that cause the differences in cancer cell dissemination. Further, we’re using an intravital imaging (IVI) and cell culture approach to define the molecular pathways involved in these cellular interactions. Finally, we’re identifying available drugs that can modify these pathways and cellular interactions in patients through clinical trials.
In 2012, you were one of a few Einstein research scientists who received federal funding to support your work. Are funding streams for translational research as sparse as they were back then or is the situation improving, or getting worse?
I was awarded a National Cancer Institute (NCI) RO1 grant totaling $1.4 million over four years to study metastatic breast cancer. This grant was part of NCI’s "Provocative Questions" program, geared toward advancing work on 24 promising but overlooked areas of cancer research.
Unfortunately, the federal funding situation for cancer research has not improved and the NCI pay line has been lingering around 8% for the past decade. This really halts innovative research because it is difficult to get funding for innovative, high- risk research and requires an enormous amount of time devoted to re-writing proposals instead of moving forward.
You published a review article that year in the Journal of Histochemistry and Cytochemistry.
That’s right. This work was done in collaboration with Drs. Joan Jones and John Condeelis. Based on Dr. Condeelis’s pre-clinical work on animal breast cancer models, Dr. Jones developed an immunohistochemical assay for detection of TMEM doorways. In our review we described a new immunohistochemical approach for the assessment of metastatic risk based on the density of cancer cell intravasation sites called TMEM (Tumor MicroEnvironment of Metastasis).
How has that research progressed?
In 2014, we published our study describing the relationship of TMEM doorways and a subpopulation of cancer cells capable of using these doorways. The majority of work was done using tissue from breast cancer patients and it heavily relied on pathology and our ability to extract caner cells by FNA from human tumors.
You and your colleagues (Karagiannis et. al.) coauthored a groundbreaking study published in Science Translational Medicine in 2017. What were the key findings?
We noticed that in certain patients who have residual disease after completion of pre-operative chemotherapy the density of TMEM doorways increases. In pre-clinical models we also showed that chemotherapy can induce pro-metastatic changes in the tumor microenvironment as well as cancer cell dissemination into the lungs. This prompted us to study the effect of chemotherapy on the tumor microenvironment in patients of different racial backgrounds.
Have you received continuation funding?
Yes, we obtained funding from the New York State Department of Health (NYS-DOH) last year to study racial disparity in the reaction of the tumor microenvironment to cancer treatment. This currently represents the backbone of the NYPOG’s focus. Dr. David Entenberg, a NYPOG member, also received NYS-DOH funding to study specific pathways involved in the induction of the cancer cell-disseminating phenotype. Moreover, Drs. Thomas Rohan and John Condeelis, both members of the NYPOG, have received R01 funding to study the prognostic power of TMEM doorway density and other TMEM dissemination-related markers. I’m extensively involved in these studies as a collaborator. In addition, just this year we submitted two R01s, one as an investigator initiated R01 and one as part of the larger Program Project Grant lead by Dr. Jonathan Backer. Both R01s are focused on studying metastasis, in particular the cellular and molecular mechanisms of disseminating cancer cell phenotypes. Hopefully, we will be successful in obtaining more funds for this important work.
In the “old days” of cancer research, the study of metastasis posed a challenge for investigators because it wasn’t possible to view the process in real time. How has technology changed that?
Metastasis is a dynamic process which requires that cells from primary tumors enter vasculature and travel to distant organs. This can only be observed using imaging of live tumors. We’re fortunate to have one of best intravital imaging (IVI) facilities in the world at Einstein, and the best experts in performing IVI. However, the mission and vision of the NYPOG extends beyond IVI; our ultimate goal is to translate the discoveries from IVI into studying patient tissues, and ultimately, into improving cancer care for all patients.
Before we wrap up, one final, very important question: What do you do for fun when you’re not working in the lab?
I enjoy spending time with my family. Due to COVID restrictions we started cooking and baking together, which turned out to be a lot of fun! As animal lovers, we cook only vegan. I also enjoy my daily yoga practice and going on long walks with my Leonberger. At the end of the day, a good fiction book or knitting by the fireplace with old hits playing in the background is amazing! Before COVID restrictions I enjoyed traveling and visiting theaters, art galleries and restaurants with family and friends and look forward to doing that again.
Thank you, Dr. Oktay.
Posted on: Tuesday, January 05, 2021

