When performing multicolor flow cytometric analysis, a major factor in the success of the analysis is the choice of which antibody to use with which fluorochrome. There are often many "correct" combinations possible. A number of factors need to be considered in making choices. For any given monoclonal antibody, the signal-to-noise ratio of positive to negative can differ four- to six-fold depending on the fluorochrome used (see below). Also, the relative fluorochrome intensity depends on the instrument. A highly expressed antigen will be resolved with almost any fluorophore. An antigen expressed at lower density might require the higher signal-to-noise ratio provided by a PE or APC conjugate to separate the positive cells adequately from the unlabeled cells.
The picture below shows an example of the staining pattern using the same monoclonal antibody conjugated to six commonly used fluorochromes and run on 2 different cytometers. This work was not performed at AECOM. The pictures exists to detail the tremendous differences that can be observed between instruments and among different fluorophores; hence, it should be used as general guideline for the relative intensities of various fluorophores run on the core's cytometers.

Picture courtesy of Becton Dickinson
SELECTED FLUOROPHORES
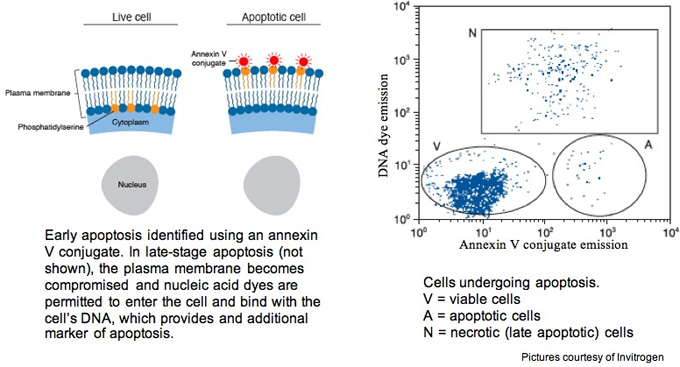
7-Aminoactinomycin D (7-AAD), like propidium iodide (discussed below), is a DNA intercalating dye but 7-AAD is specific for cytosine-guanine base pairs. It is well suited for viability measurements and also for apoptosis experiments where it's paired with annexin V conjugates. During early apoptosis, phosphatidylserine is translocated from the cytoplasmic face of the plasma membrane to the external face. Annexin V, when in the presence of Ca2+, has a high affinity for exposed cytoplasmic phosphatidylserine. Unlike propidium iodide, this dye has minimal emission bleed from the FL3 detector into the FL2 (PE) detector on the FACSCalibur or FACScan. Whereas PI can be detected in either FL2 or FL3, though it is typically detected in FL2, of the FACScan or FACSCalibur, 7-AAD is detected in FL3.
Alexa Fluor 488 has a spectrum almost identical to that of fluorescein isothiocyanate (FITC), but with extraordinary photostability. Because of this photostability, it has become a choice for fluorescent microscopy applications and has become popular in cytometry applications. It is detected in the FL1 detector of a FACSCalibur or FACScan. Unlike other fluorochromes with similar emission spectra, Alexa Fluor 488 is pH insensitive over a broad range.
Alexa Fluor 633 is a practical alternative to APC as well as Cy5. Alexa Fluor 633 conjugates can be used in multi-color flow cytometry with instruments equipped with a second red laser or red diode. It is detected in the FL4 detector of the core's upgraded 2-laser FACScans. Like other Alexa Fluor dyes, Alexa Fluor 633 exhibits uncommon photostability, making it an ideal choice for fluorescent microscopy.
A great number of different Alexa Fluor dyes exist that are beyond the scope of this introductory fluorophore section. Many manufacturers sell directly-conjugated Alexa Fluor antibodies. Know that Molecular Probes' Zenon Antibody Labeling Kits, which are available for all of their Alexa Fluor dyes, make it possible to rapidly and quantitatively label antibodies from a purified antibody fraction or from a crude antibody preparation such as serum, ascites fluid or a hybridoma supernatant.
Allophycocyanin (APC) is an accessory photosynthetic pigment found in blue-green algae. APC has 6 phycocyanobilin chromophores per molecule, which are similar in structure to phycoerythrobilin, the chromophore in phycoerythrin or PE. APC tandem dyes, APC-Cy5.5 and APC-Cy7, are also available. APC has a 650-nanometer wavelength absorption maximum and a 660-nanometer fluorescence emission maximum. APC can be used in flow cytometers equipped with dual lasers for multi-color analysis. Like Alexa Fluor 633, APC is excited using the helium-neon red diode laser (633 nanometers) of the core's upgraded 2-laser FACScans and is detected using the FL4 detector.
APC-Cy7 (also written Cy7-APC) is a tandem conjugate system that combines APC and a cyanine dye (Cy7) and has an absorption maximum at ~650 nanometers. This tandem uses the efficiency of the fluorescence light energy transfer between the two fluorochromes. When excited by light from a helium-neon laser, the excited fluorochrome (APC) is able to transfer its fluorescent energy to the cyanine molecule, which then fluoresces at a longer wavelength. The resulting fluorescent emission maximum is in the deep red at approximately 767 nanometers. APC-Cy7 conjugates cannot be detected on a FACSCalibur because the FL4 detector's optical filter is centered for APC emission (660 nanometers) and not the longer red emission excited with a helium-neon laser, however, the core facility's upgraded 2-laser FACScans can detect this conjugate because the standard FL4 detector's optical filter was replaced with a long pass optical filter. It is recommended that special precautions be taken with this conjugate, and cells stained with them, to protect the fluorochrome from long-term exposure to visible light.
Autofluorescence
Autofluorescence is a major concern in basic research flow cytometry environments and no section on fluorophores can be considered complete without recognizing the role that cellular autofluorescence plays. Individual cell populations have characteristic levels of autofluorescence (fluorescent signals generated by the cells themselves). Mammalian cellular autofluorescence, at least in lymphocytes, comes predominantly from pyridine and flavin nucleotides. Pyridine nucleotides are excited most efficiently by UV light (~340 nm) and have an emission maximum in the blue at 450-470 nm. Flavin nucleotides are the primary issue in flow cytometry laboratories because those molecules are excited in the cyan-blue range (430-500 nm) of the color spectrum, which is where flow cytometer's primary lasers emit light (488 nm). When excited, flavin nucleotide's emission (530-550 nm) is the same emission range as FITC/eGFP (green), PE (orange) and, to a lesser extent, PE-Cy5/PerCP (red emission) and PE-Cy7 (far red emission). The take-home message is that while autofluorescence is observed in all fluorescence channels, it decreases dramatically at longer wavelengths (>600 nanometers).
- For cell types that exhibit high levels of autofluorescence, using a dye with a longer emission wavelength (e.g., APC, APC-Cy7, etc.) often provides an excellent signal-to-noise ratio.
- For cell types that are not very autofluorescent, such as fresh cells, the improved separation seen with long-wavelength excitation is less apparent. In those cases, conjugates such as FITC and PE can be used.
Carboxyfluorescein Diacetate (CFSE) can be used to track asynchronous cell division. Cell division results in sequential halving of the initial fluorescence, resulting in a cellular fluorescence histogram. CFSE has a green emission that's collected in the FL1 detector of a FACScan.
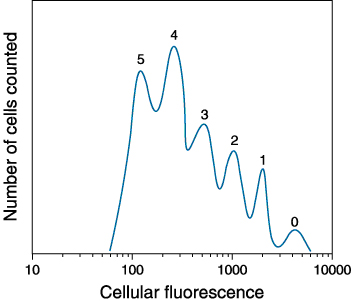
The peaks labeled 1, 2, 3, 4 and 5 represent successive generations of CFSE-cultured cells. Picture courtesy of Molecular Probes.
Cy3 and Cy5 are excited by the 488-nanometer line of an argon laser and the 633-nanometer line of a helium-neon diode laser, respectively. These conjugates can be used in flow cytometry but typically do not give the fluorescence intensity comparable to that of PE or APC. Applications where a smaller dye is required are more appropriate for these dyes. These fluorochromes are well suited for fluorescent microscopy.
Cyan Fluorescent Protein (eCFP) cannot be analyzed using a FACScan or FACSCalibur as this molecule requires excitation in the violet range. The protein is easily detected using the violet lasers (407nm) that exist on all of the other flow cytometers in the facility. This molecule has an excitation maximum at 475 nanometers and is best excited using the MoFlo's 457nm line of the argon or argon-krypton mixed gas laser. More detailed discussion of this molecule can be found in the next section of this web site, the section on Fluorescent Proteins.
Fluorescein isothiocyanate (FITC) is currently the most commonly used fluorescent dye for flow cytometry analysis. When excited at 488 nanometers, FITC has a green emission that's usually collected at 530 nanometers, the FL1 detector of a FACSCalibur or FACScan. FITC has a high quantum yield (efficiency of energy transfer from absorption to emission fluorescence) and approximately half of the absorbed photons are emitted as fluorescent light. For fluorescent microscopy applications, FITC is seldom used as it photobleaches rather quickly though in flow cytometry applications, its photobleaching effects are not observed due to a very brief interaction at the laser intercept. FITC is highly sensitive to pH extremes.
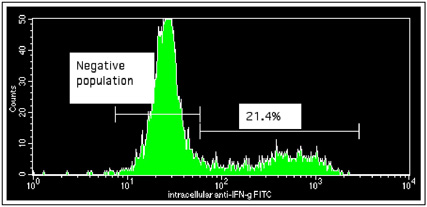
Purified T cells were stimulated in culture concurrent with a reagent that inhibits intracellular transport and the cell membranes were subsequently fixed and permeabilized. Cells were then labeled with a gamma interferon antibody conjugated to FITC. Gamma interferon production is used to analyze type 1 immune responses.
Fluorescein Proteins (see eCFP, eGFP and eYFP, listed in this section, as well as the next section of this web site)
Green Fluorescent Protein (eGFP) can be excited at 488 nanometers with a peak emission at 509 nanometers and is detected in the FL1 detector on the FACSCalibur or FACScan. The LSRII and all of the core's sorter flow cytometers are able to distinguish between concurrently expressing eGFP and eYFP cells when the proper optical filters and experimental controls exist. More detailed discussion of this molecule can be found in the next section of this web site, the section on Fluorescent Proteins.
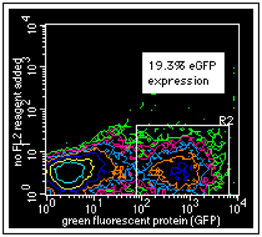
Mouse neurons were transfected with a β-actin promotor that drives the expression of an eGFP gene. Forty hours after transfection, cells were harvested in order to determine the transfection efficiency.
Peridinin chlorophyll protein (PerCP) has a 677 nanometer maximum emission, red, when excited at 488 nanometers and is detected on the FL3 detector of a FACSCalibur or FACScan. A PerCP tandem dye is also available (PerCP-Cy5.5, also written Cy5.5-PerCP). PerCP is not suited for the high-powered lasing (>150mW) applications, such as on a MoFlo, due to its photobleaching characteristics. PerCP conjugates can only be obtained from the Becton Dickinson/Pharmingen conglomerate.
Phycoerythrin (PE or R-PE) has a huge absorption coefficient and almost perfect quantum efficiency. In vivo, it functions to transfer light energy to chlorophyll during photosynthesis. It is one of the brightest dyes used today and emits in the yellow/orange at about 570 nanometers. Those accustomed to fluorescent microscopy may not be familiar with this fluorochrome as it photobleaches rather quickly under a microscope.
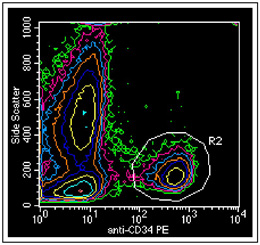
CD34 antibody conjugated to PE and used to determine the quantity of stem cells contained in a donor marrow for transplant.
Phycoerythrin-Cy5 (PE-Cy5, also written Cy5-PE) is a tandem conjugate where PE is coupled to the cyan dye, Cy5. When excited by 488-nanometer light, the excited fluorochrome (PE) is able to transfer its fluorescent energy to the cyanine molecule, which then fluoresces at a longer wavelength in the red at 670 nanometers. This tandem dye is known by a confusing myriad of names to include:
- Becton Dickinson's CyChrome
- Caltag and Sigma's Tri-Color
- Coulter's PC5
- GIBCO's RED670 and probably others.
Other PE conjugates exist, e.g., PE-Cy5.5 and PE-Cy7, that will not be discussed in this introductory fluorophore section. It is recommended that special precautions be taken with this conjugate, and cells stained with them, to protect the fluorochrome from long-term exposure to visible light.
Phycoerythrin-Texas Red (PE-Texas Red, also written Texas Red-PE) is a tandem conjugate where PE is coupled to Texas Red. Similar to other tandem conjugates, when excited by 488-nanometer light, the excited fluorochrome (PE) is able to transfer its fluorescent energy to the Texas Red molecule, which then fluoresces at a longer wavelength with a peak in the orange at 612 nanometers. This tandem is also known by other names such as:
- Red 612
- ECD (Electron Coupled Dye)
Know that PE-Texas Red conjugates run on a FACScan or FACSCalibur will result in dull expression due to the extant optical filters. This is not observed on the other cytometers in the facility when they are equipped with the appropriate optical filters for this conjugate. There is considerable overlap of emission when running PE and PE-Texas Red specimens.
Propidium Iodide (PI) is a membrane-impermeant dye that stains by nondiscriminately intercalating into every 4th or 5th nucleic acid base pair, binding both DNA and RNA. Once bound, PI undergoes a conformational change and becomes ~40 times brighter. Propidium iodide has a broad emission spectrum with a peak in the orange at 620 nanometers. A number of assays employ propidium iodide. Cells or nuclei with altered DNA content are identified by staining alcohol-fixed, RNAse-treated cells or nuclei with this dye (see below).
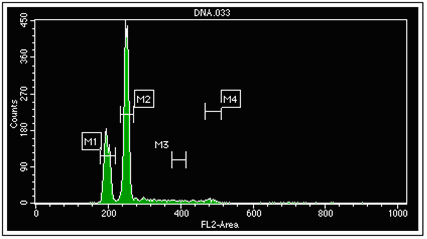
The first peak (M1) on the left represents normal diploid cells. The next major peak (M2) represents tumor cells with increased DNA content and DNA index of 1.27. Propidium iodide can be combined with additional fluorescent antibodies that are specific for a unique cell population and allow for more accurate S phase analysis of multiple overlapping populations.
Propidium iodide has also been employed for many years as a marker for viability as the disrupted membranes of dead cells allow the dye to pass freely to the nucleic acids. However, this dye is very sticky; it will stick to sample tubing and, given sufficient time exposure to living cells, living cells will appear to be propidium iodide positive. Given this dye's broad emission spectrum and its sticky properties, contemporary flow cytometry labs have replaced propidium iodide with other nucleic acid dyes, e.g., 7-AAD (listed below), TO-PRO-3, or DAPI, among many others, for viability measurements though propidium iodide remains the most commonly used dye for DNA content analysis.
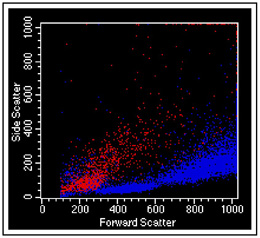
In a viability test, cells labeled with propidium iodide are color-coded red, live cells are colored blue. Laser light scatter by itself is oftentimes sufficient to discern dead cells from live ones. The small blue particles in the lower left are small bits of debris that do not contain any DNA.
Texas Red has an excitation maximum in the yellow-orange range of the color spectrum; consequently, Texas Red cannot be excited on any of the benchtop analyzers in the core facility. It can, however, be detected using a yellow laser of all 4 of core's sorter flow cytometers. When excited, Texas Red has an excitation maximum in the orange at 612 nanometers.
Yellow Fluorescent Protein (eYFP), a yellow-shifted variant of the eGFP molecule, is also excited at 488 nanometers with a peak emission at 535 nanometers and is also detected in the FL1 detector on a FACSCalibur or FACScan. More detailed discussion of this molecule can be found in the next section of this web site, the section on Fluorescent Proteins.