The Flow Cytometry Core Facility is equipped with 6 flow cytometers that are categorized into 2 types:
-
Bench top analyzer flow cytometers that are operated by the investigators themselves and
-
High-speed cell sorter flow cytometers that are operated by the core facility's personnel as a service to investigators only.
The flow cytometers in the facility are equipped with an amazing variety of laser excitation wavelengths, which are shown in the following table:
|
Laser Lines in the Flow Cytometry Core Facility at Einstein
|
| |
High Speed Cell Sorter Flow Cytometers |
Bench Top Analyzer Flow Cytometers |
Laser Scanning Cytometer |
| Laser Line (nm) |
BigFoot |
MolFlowXDP |
FACSAria |
Aurora |
LSRIIs |
CantoII |
iCys |
| UV (349/355) |
√ |
|
|
√ |
√ |
|
|
| Violet (405) |
√ |
√ |
√ |
√ |
√ |
|
√ |
| Cyan (445) |
√ |
|
|
|
|
|
|
| Blue (488) |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
| Green (514-532) |
|
|
|
|
|
|
|
| Yellow (561) |
√ |
√ |
√ |
√ |
√ |
|
|
| Orange (592) |
|
|
|
|
|
|
|
| Red (640) |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
| Far Red (676) |
|
|
|
|
|
|
|
| Infrared (785) |
√ |
|
|
|
|
|
|
Core Facility Bench Top Analyzer Flow Cytometers:
There are 5 bench top analyzer flow cytometers in the core. Two five laser Auroras with auto sampler from Cytek, two BD LSRIIs, one CantoII from BD Bioscience.
Two Cytek Auroras are full spectrum analyzers. With five lasers( 355,405, 488, 561 and 640nm), three scattering channels, and 64 fluorescence channels, the Aurora system suits every laboratory’s needs, from simple to high-complexity applications. It is able to handle more than 40 color panels. The state-of-the-art optics and low-noise electronics provide excellent sensitivity and resolution. Flat-top laser beam profiles, combined with a uniquely designed fluidics system, translate to outstanding performance at high sample flow rates.

Available Dyes in the Corresponding Channels:
|
UV Excited Fluors
|
Peak Channel
|
| BUV395 |
UV2 |
| BUV496 |
UV7 |
| BUV563 |
UV9 |
| BUV661 |
UV11 |
| BUV737 |
UV14 |
| BUV805 |
UV16 |
|
Violet Excited Fluors
|
Peak Channel
|
| BV421 |
V1 |
| Alexa Fluor 405, SuperBright 436 |
V2 |
| eFluor450 , VioBlue, Pacific Blue |
V3 |
| BV480 |
V4 |
| eFluor 506 |
V5 |
| BV510, VioGreen |
V7 |
| BV570, Pacific Orange |
V8 |
| BV605, SuperBright 600, Qdot 605 |
V10 |
| BV650, SuperBright 645, Qdot 655 |
V11 |
| BV711, SuperBright 702, Qdot705 |
V13 |
| BV750 |
V14 |
| BV785, BV786, Qdot 800 |
V15 |
|
Blue Excited Fluors
|
Peak Channel
|
| Vio 515, sVio 515, BB515 |
B1 |
| Alexa Fluor 488, FITC, VioBright FITC |
B2 |
| Alexa Fluor 532 |
B3 |
| PerCP |
B8 |
| PerCP/Cy5.5, BB700 |
B9 |
| PerCP-eFluor 710, PerCP-Vio 700 |
B10 |
|
Yellow Green Excited Fluors
|
Peak Channel
|
| PE |
YG1 |
| PE/Dazzle 594, PE-CF594, PE-TexasRed, PE-eFluor 610 |
YG3 |
| PE-Alexa Fluor 610 |
YG4 |
| PE/Cy5 |
YG5 |
| PE-Cy5.5, PE-AlexaFluor 700 |
YG7 |
| PE/Cy7, PE-Vio 770 |
YG9 |
|
Red Excited Fluors
|
Peak Channel
|
| APC |
R1 |
| Alexa Fluor 647, Vio 667, sVio 667, efluor660 |
R2 |
| APC-Cy5.5 |
R3 |
| Alexa Fluor 700, APC-R700 |
R4 |
APC-Alexa750, APC/Fire 750, APC-Cy7
APC-Vio 770, APC-efluor780, APC-H7 |
R7 |
Available Detectors:
|
Laser
|
Channel
|
Center Wavelength (nm)
|
Bandwidth (nm)
|
Wavelength Start (nm)
|
Wavelength End (nm)
|
| Ultraviolet |
UV1 |
372 |
15 |
365 |
380 |
| UV2 |
387 |
15 |
380 |
395 |
| UV3 |
427 |
15 |
420 |
435 |
| UV4 |
443 |
15 |
435 |
450 |
| UV5 |
458 |
15 |
450 |
465 |
| UV6 |
473 |
15 |
465 |
480 |
| UV7 |
514 |
28 |
500 |
528 |
| UV8 |
542 |
28 |
528 |
556 |
| UV9 |
581 |
31 |
566 |
597 |
| UV10 |
612 |
31 |
597 |
628 |
| UV11 |
664 |
27 |
650 |
677 |
| UV12 |
691 |
28 |
677 |
705 |
| UV13 |
720 |
29 |
705 |
734 |
| UV14 |
750 |
30 |
735 |
765 |
| UV15 |
780 |
30 |
765 |
795 |
| V16 |
812 |
34 |
795 |
829 |
|
Laser
|
Channel
|
Center Wavelength (nm)
|
Bandwidth (nm)
|
Wavelength Start (nm)
|
Wavelength End (nm)
|
| Violet |
V1 |
428 |
15 |
420 |
435 |
| V2 |
443 |
15 |
436 |
451 |
| V3 |
458 |
15 |
451 |
466 |
| V4 |
473 |
15 |
466 |
481 |
| V5 |
508 |
20 |
498 |
518 |
| V6 |
525 |
17 |
516 |
533 |
| V7 |
542 |
17 |
533 |
550 |
| V8 |
581 |
19 |
571 |
590 |
| V9 |
598 |
20 |
588 |
608 |
| V10 |
615 |
20 |
605 |
625 |
| V11 |
664 |
27 |
651 |
678 |
| V12 |
692 |
28 |
678 |
706 |
| V13 |
720 |
29 |
706 |
735 |
| V14 |
750 |
30 |
735 |
765 |
| V15 |
780 |
30 |
765 |
795 |
| V16 |
812 |
34 |
795 |
829 |
|
Laser
|
Channel
|
Center Wavelength (nm)
|
Bandwidth (nm)
|
Wavelength Start (nm)
|
Wavelength End (nm)
|
| Blue |
B1 |
508 |
20 |
498 |
518 |
| B2 |
525 |
17 |
516 |
533 |
| B3 |
542 |
17 |
533 |
550 |
| B4 |
581 |
19 |
571 |
590 |
| B5 |
598 |
20 |
588 |
608 |
| B6 |
615 |
20 |
605 |
625 |
| B7 |
660 |
17 |
652 |
669 |
| B8 |
678 |
18 |
669 |
687 |
| B9 |
697 |
19 |
688 |
707 |
| B10 |
717 |
20 |
707 |
727 |
| B11 |
738 |
21 |
728 |
749 |
| B12 |
760 |
23 |
749 |
772 |
| B13 |
783 |
23 |
772 |
795 |
| B14 |
812 |
34 |
795 |
829 |
|
Laser
|
Channel
|
Center Wavelength (nm)
|
Bandwidth (nm)
|
Wavelength Start (nm)
|
Wavelength End (nm)
|
| Yellow Green |
YG1 |
577 |
20 |
567 |
587 |
| YG2 |
598 |
20 |
588 |
608 |
| YG3 |
615 |
20 |
605 |
625 |
| YG4 |
660 |
17 |
652 |
669 |
| YG5 |
678 |
18 |
669 |
687 |
| YG6 |
697 |
19 |
688 |
707 |
| YG7 |
720 |
29 |
706 |
735 |
| YG8 |
750 |
30 |
735 |
765 |
| YG9 |
780 |
30 |
765 |
795 |
| YG10 |
812 |
34 |
795 |
829 |
|
Laser
|
Channel
|
Center Wavelength (nm)
|
Bandwidth (nm)
|
Wavelength Start (nm)
|
Wavelength End (nm)
|
| Red |
R1 |
660 |
17 |
652 |
669 |
| R2 |
678 |
18 |
669 |
687 |
| R3 |
697 |
19 |
688 |
707 |
| R4 |
717 |
20 |
707 |
727 |
| R5 |
738 |
21 |
728 |
749 |
| R6 |
760 |
23 |
749 |
772 |
| R7 |
783 |
23 |
772 |
795 |
| R8 |
812 |
34 |
795 |
829 |
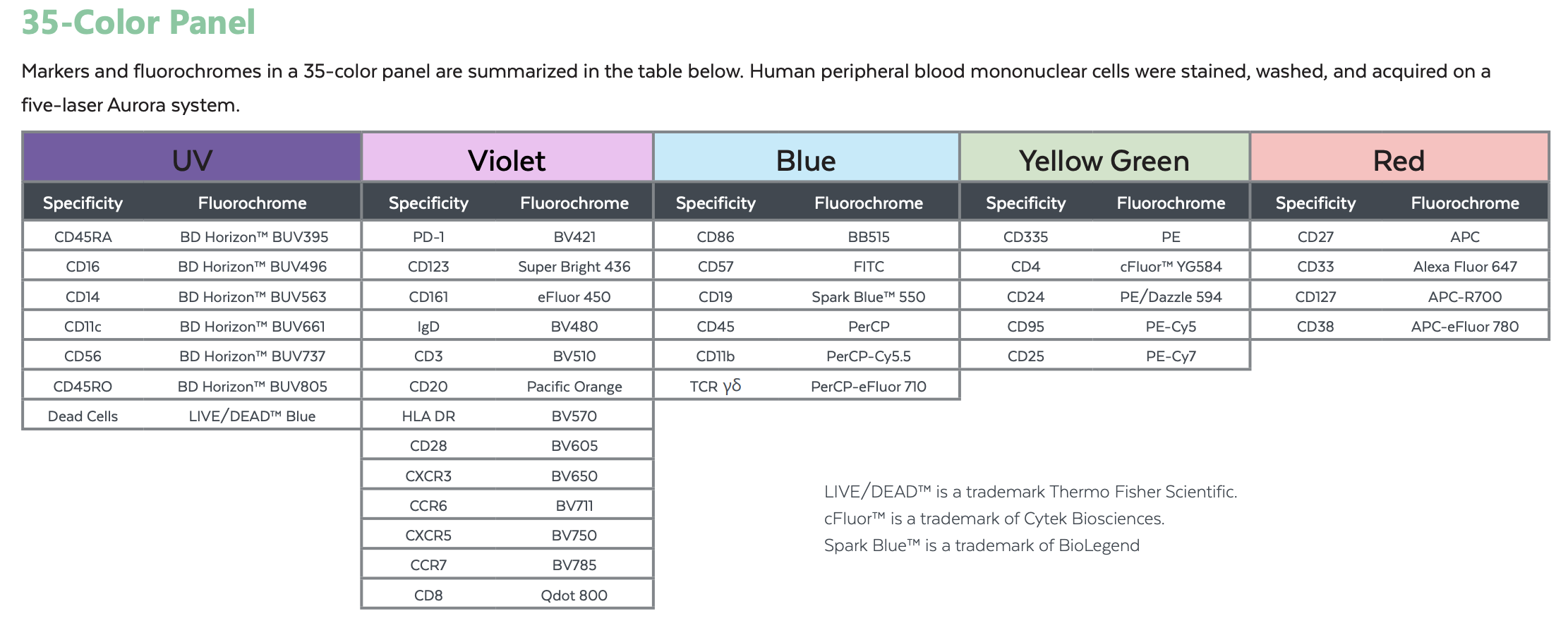
A 35 Color Example:
Human peripheral blood mononuclear cells were stained, washed, and acquired on a five-laser Aurora system
Suggested 23 Dye Combination:
Becton Dickinson LSRII-Yellow: The BD™ LSR II flow cytometer is the most flexible yet powerful benchtop analyzer available. Innovative technology in the BD LSR II optics and digital electronics have created a more sensitive flow cytometer that yields more information from each sample.

The second LSRII we purchased recently has 5 lasers, 355nm, 405nm, 488nm, 561nm and 640nm. It can analyze up to 14 colors simultaneously.
|
LSRII Yellow Configuration
|
| Laser |
Detector Name |
Bandpass Filter |
Dichoric Filter |
Fluorochrome Detected |
| Blue Laser (488 nm) |
A |
710/50 |
685 DLP |
PerCP-Cy5.5, PerCP |
| B |
575/26 |
550 DLP |
PI |
| C |
525/50 |
505 DLP |
FITC, GFP, YFP |
| D |
488/10 |
Blank |
SSC |
| Red Laser (640 nm) |
A |
780/60 |
750 DLP |
APC-Cy7 |
| B |
730/45 |
685 DLP |
Alex700 |
| C |
660/20 |
Blank |
APC, Alex647 |
| Yellow Laser (561 nm) |
A |
780/60 |
750 DLP |
PE-Cy7 |
| B |
670/30 |
645 DLP |
PE-Cy5 |
| C |
610/20 |
600 DLP |
PE-TxRed-YG, Mcherry |
| D |
582/15 |
Blank |
PE-YG, RFP |
| violet Laser (405 nm) |
A |
525/50 |
505 DLP |
Am Cyan |
| B |
450/50 |
Blank |
Pacific Blue |
| UV Laser (355 nm) |
A |
530/30 |
505 DLP |
Indo-1 (blue) |
| B |
450/50 |
Blank |
DAPI, Indo-1 (Violet) |
Becton Dickinson LSRII-U: We recently add a 561nm Yellow laser to this LSRII.

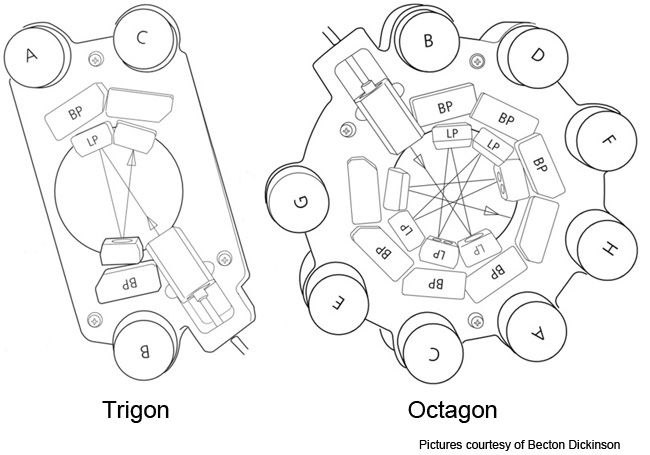
The LSRII-U has optical detectors and removable optical filters that can permit the detection of different fluorophores and fluorophore combinations than are regularly used on the FACSCalibur. The core's LSRII-U has 5 lasers with 2 detectors for laser-light scatter and 13 detectors for fluorescence in the following configuration:
- a 20 milliwatt solid-state argon laser that emits blue light at 488 nanometers and which is connected to a unique Trigon optical detector 'block' that's equipped with 3 detectors (2 fluorescence and 1 laser-light scatter), which are detailed below
- a 40 milliwatt solid-state laser that emits red light at ~640 nanometers and is connected to a unique trigon optical detector block that's equipped with 3 fluorescence detectors
- a 20 milliwatt laser solid-state UV that emits non-visible ultra-violet light at 350 nanometers and which is also connected to trigon optical detector block that's equipped with 2 fluorescence detectors
- a 100 milliwatt solid-state violet laser that emits violet light at 407 nanometers and which is connected to trigon optical detector block that's equipped with 2 fluorescence detectors
- a 50 milliwatt solid-state yellow laser that emits red light at ~561 nanometers and is connected to a unique Octagon optical detector block that's equipped with 4 fluorescence detectors
LSRII-U optical configuration:
|
LSRII-U Configuration
|
| Laser |
Detector Name |
Bandpass Filter |
Dichoric Filter |
Fluorochrome Detected |
| Blue Laser (488 nm) |
A |
695/40 |
685 DLP |
PerCP-Cy5.5, PerCP |
| B |
530/30 |
505 DLP |
FITC, GFP, YFP |
| C |
488/10 |
Blank |
SSC |
| Red Laser (640 nm) |
A |
780/60 |
735 DLP |
APC-Cy7 |
| B |
710/20 |
685 DLP |
Alex700 |
| C |
670/30 |
Blank |
APC, Alex647 |
| Yellow Laser (561 nm) |
A |
780/60 |
735 DLP |
PE-Cy7 |
| B |
670/14 |
635 DLP |
PE-Cy5 |
| C |
610/20 |
600 DLP |
PE-TxRed-YG, Mcherry |
| D |
586/15 |
Blank |
PE-YG, RFP |
| violet Laser (405 nm) |
A |
525/50 |
505 DLP |
Am Cyan |
| B |
440/40 |
Blank |
Pacific Blue |
| UV Laser (355 nm) |
A |
530/30 |
505 DLP |
Indo-1 (blue) |
| B |
440/40 |
Blank |
DAPI, Indo-1 (Violet) |
Becton Dickinson FACS CantoII: The BD FACSCanto II features many innovations for both clinical and research labs, including a true fixed-alignment flow cell to minimize startup time and improve reproducibility.
With High Throughput Sampler (HTS) option available for this flow cytometer. The HTS provides fully automated and rapid sample acquisition from either a 96- or 384-well microtiter plate.

|
FACS CantoII Configuration
|
| Laser |
PMT position |
BP Filter |
LP mirror |
Intended Dye |
| 488 nm |
A |
780/60 |
735DLP |
Pr-Cy7 |
| B |
670LP |
655DLP |
PerCP, PerCP-Cy5.5 |
| C |
Blank |
610DLP |
--- |
| D |
585/42 |
556DLP |
PE, PI |
| E |
530/30 |
502DLP |
FITC, Alex488 |
| 633 nm |
A |
780/60 |
735DLP |
Apc-Cy7, Alex750, Alex780, APC-eFluor 750 |
| B |
Blank |
685DLP |
|
| C |
660/20 |
|
APC, Alex633, Alex647, To-PRO-3 |
ThermoFisher BigFoot Spertrum Cell sorter: Flexibly designed by cell sorting experts for a broad range of sorting applications
The Bigfoot Spectral Cell Sorter is designed to expand your reach while fitting your workflow. With 7 lasers ( 349, 405, 445, 488, 561, 640, and 785nm) and 60 detectors, you can run your existing experiments using conventional compensation, or take advantage of the spectral unmixing capabilities to resolve even more cell populations. Integrated biocontainment and aerosol management help protect you and your samples.
With sort rates exceeding 70,000 events per second, the system can sort a 96-well plate in less than 8 seconds and a 384-well plate in less than 11 seconds. Simple enough for an individual lab, and robust enough for a core facility, the Bigfoot Spectral Cell Sorter will help you master the full range of sorting experiments, from sorting cells labeled with fluorescent proteins to deep immunophenotyping, genomics, cell and gene therapy research, and other high-performance, high-throughput applications.

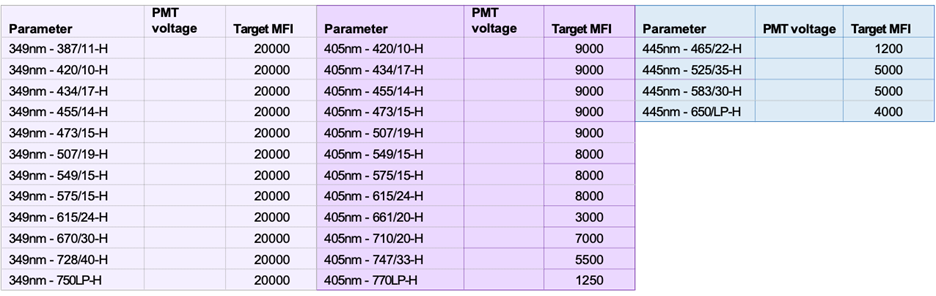
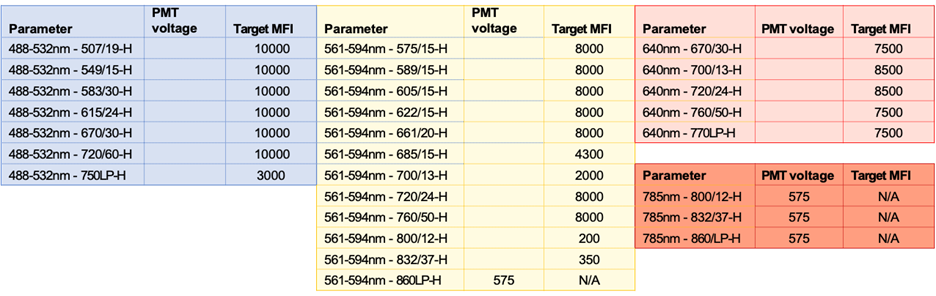
Target MFI Recommendations and Filters for each laser: 349, 405, 445, 488, 561, 640 and 785 nm Lasers:
A 35 Color Example:
Human peripheral blood mononuclear cells were stained, washed, and acquired on a five-laser Aurora system.
Core Facility High-Speed Cell Sorter Flow Cytometers:
There are 3 high-speed cell flow cytometers in the core facility that consist of 3 different models by 2 manufacturers. The facility is equipped with a DakoCytomation MoFlo and a Dako (formerly DakoCytomation) MoFlo XDP. Dako is based in Fort Collins, Colorado. The 3rd sorter in the facility is a Becton Dickinson FACSAria.
The primary function of the high-speed cell sorter flow cytometers are for physically sorting cells or particles of interest and for analysis requirements that cannot be met on the bench top analysis flow cytometers in the facility. For example, the sorter flow cytometers are the only flow cytometers in the facility that are equipped with cyan, green, yellow, orange or far red laser lines, consequently, investigators requiring access to these excitation lines are granted access to the sorter flow cytometers regardless if their goal is to physically sort cells (particles) of interest, otherwise, as stated previously, the primary function of the sorter flow cytometers is for physically sorting cells or particles of interest.
Unlike the bench top analyzer flow cytometers, which are operated by the investigators themselves, sorter operation is provided as a service. This is because the instruments are much more complex than the bench top analyzers and consequently require much more experience and training to achieve proper function. Also, because of the open design of the MoFlos, which permits great flexibility in experimental design, their components are more easily damaged and they are expensive to replace.
Because the sorters use a stream-in-air sorting method, they aerosolize the samples. Consequently, the sorters cannot be used for sorting hazardous samples. Non-hazardous living cells can be sorted and recovered in sterile form for subsequent culture or for in vitro functional studies. Accommodations can be made for AECOM investigators requiring sorting of hazardous specimens off site - please inquire in advance.
Dako MoFlo XDP high-speed cell sorter flow cytometer [12-color, 4-laser system with optional 4-way sorting, aerosol containment, cloning option and sample station/sort receptacle heater/chiller]; available as a service with online booking.
The MoFlo XDP - AECOM's was the first commercial installation of this model flow cytometer in the world - is almost identical in 'architecture' to the core's 'original' (existing) MoFlo. The 2 MoFlos have the identical optical pathways and fluidic systems though the MoFlo XDP is equipped with 12, not 8, fluorescent detectors. The primary difference between AECOM's 2 MoFlos is in the electronics where the MoFlo XDP uses completely digital processing whereas the original MoFlo uses analog-to-digital processing though there is no difference in their sorting speed. The fluorescent detectors are a newer generation than those that exist in the existing MoFlo and the company claims that the XDP is more sensitive than the original MoFlo. Perhaps the most notable difference between the 2 MoFlos is that the MoFlo XDP is equipped with 4, not 3, lasers and, as mentioned previously, there are 12, not 8, fluorescent detectors.
The MoFlo XDP has 3 high-powered tunable-wavelength lasers, which consist of an argon, a krypton and an argon-krypton mixed-gas laser (virtually identical as the original MoFlo) though, unlike the 'original' MoFlo, the MoFlo XDP is also equipped with a high-powered 592nm (orange) fiber laser, which shares the mixed-gas laser pathway. Investigators that are interested to utilize the orange fiber laser must forgo concurrent use of the mixed-gas laser. The MoFlo XDP's lasers are detailed in the following table.
Like the original MoFlo, the MoFlo XDP has detectors and removable optical filters that can permit the detection of different fluorophores and fluorophore combinations than are regularly used on the bench top analyzer cytometers, such as mCherry and Texas Red (yellow laser excitation) as well as the newly published far-red fluorescent proteins mKate and Katushka (orange laser excitation), among many other fluorophores. As with the original MoFlo, there are other important differences between this cytometer and the bench top analyzer flow cytometers in the facility. Among them are:
- The MoFlo XDP, like all jet-in-air sorters, generally has less sensitivity for immunofluorescence than bench top analyzer flow cytometers.
-
The MoFlo XDP can also physically sort up to four populations concurrently and it is able to sort them into a great variety of collection devices:
- Microscope slides,
- Microtubes,
- 12 x 75 mm polypropelyne test tubes,
- 14, 15 or 50 ml polypropelyne tubes,
- Multiwell plates (24-, 96-, or 384-well plates, etc.; 60- or 72-well Terasaki, etc.),
- A choice of a variety of custom devices that are only limited by the space constraints within the sort chamber area.
As mentioned previously, the MoFlo XDP has the same architecture and the 'original' MoFlo in the facility though the MoFlo XDP has 12, not 8, fluorescent detectors in the following configuration for immunophenotyping:
- The tunable argon laser is typically tuned to its 488-nanometer line (blue) and the argon laser is typically placed into the primary laser pathway which contains 7, not 6, optical detectors (2 optical detectors for laser light scatter and 5, not 4, for fluorescence detection),
- The tunable krypton laser is typically tuned to its multi-line violet mode (concurrent 407-, 413- and 415-nanometer lasing) and the krypton laser is typically placed into the secondary laser pathway that contains 3, not 2, fluorescence detectors and
- The tunable argon-krypton mixed-gas laser is typically tuned to its red 647-nanometer line and the mixed-gas laser is typically placed into the tertiary laser pathway that also contains 4, not 2, optical detectors. As mentioned previously, the MoFlo XDP is also equipped with a high-powered 592nm (orange) fiber laser, which shares the mixed-gas laser pathway.
Becton Dickinson FACSAria high-speed cell sorter flow cytometer [12 color, 4 laser system with optional 4-way sorting, aerosol containment, cloning option and sample station/sort receptacle heater/chiller]; available as a service with online booking.

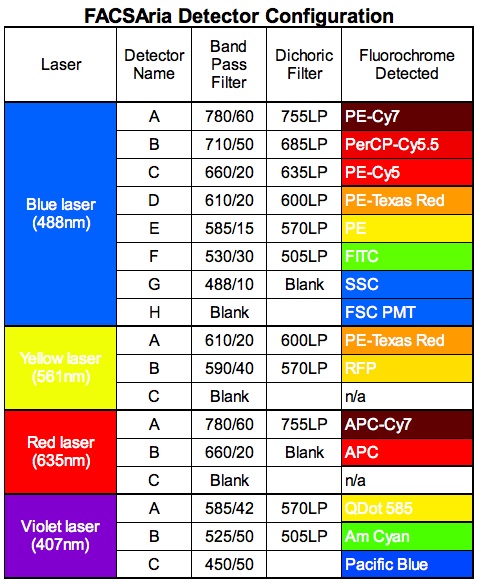
The core's FACSAria is an upgraded, 'Special Order' system that differs from the standard factory configuration that is equipped with 10 fluorescent detectors and 3 lasers. The core's FACSAria is equipped with 12, not 10, fluorescent detectors and 4, not 3, solid-state lasers as detailed in the following table. The core's FACSAria is equipped with an optional forward scatter photomultiplier tube (PMT) which is in consideration of investigators interested to sort particles <1 micron in size. The forward scatter PMT is in addition to the standard forward scatter diode, which exists in all the cytometers in the facility.
- The FACSAria, because it is a closed-cuvette sorter, has sensitivity equal to that observed in bench top analyzer flow cytometers, which is typically greater than the MoFlos.
-
Like the MoFlos, the FACSAria can physically sort up to four populations concurrently and it is able to sort them into a great variety of collection devices:
- Microscope slides,
- Microtubes,
- 12 x 75 mm test tubes,
- 15 conical tubes,
- Multiwell plates (24-, 96-, or 384-well plates, etc.; 60- or 72-well Terasaki, etc.),
The FACSAria is equipped with the following lasers in the detector configuration as described:
- Coherent Sapphire 20 milliwatt diode-pumped solid-state laser that emits blue light at 488 nanometers and which is connected to a detector bank configured in the octagonal configuration as the LSRII (see LSRII description). The FACSAria contains 8 PMTs (6 for fluorescence and 2 for laser light scatter) in the octagon.
- Coherent CUBE 40 milliwatt solid-state diode laser that emits red light at 638 nanometers and which is connected to a trigon that contains 2 fluorescent PMTs.
- Coherent CUBE 50 milliwatt solid-state diode laser that emits violet light at 405 nanometers and which is connected to a trigon that contains 3 fluorescent PMTs.
- Coherent Compass 40 milliwatt diode-pumped solid-state laser that emits yellow light at 561 nanometers and which is connected to a trigon that contains 2 fluorescent PMTs, all of which is detailed in the following table:
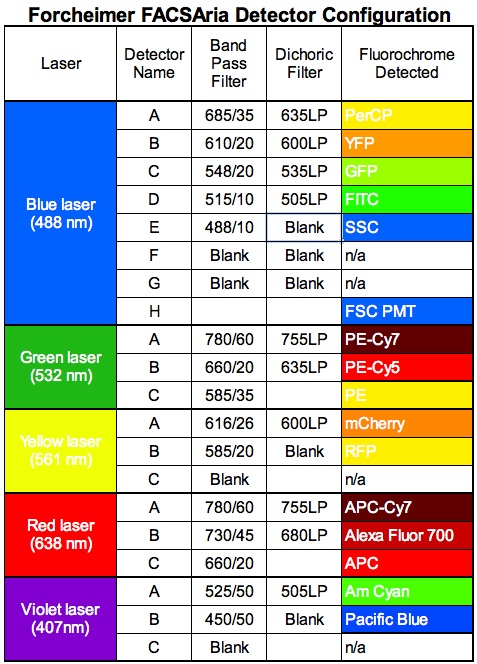
A 14-color, 5-laser FACSAria is available to investigators that is not physically located in the core facility though it is under the auspices and oversight of the core's administration and staff. The 5-laser FACSAria is equipped with the almost the identical lasers and forward scatter PMT that the 4-laser FACSAria has but the 5-laser FACSAria is also equipped with a green 532 nanometer laser. The lasers are and associated optics are:
- Coherent Sapphire 100 milliwatt diode-pumped solid-state laser that emits blue light at 488 nanometers and which is connected to an octagon that contains 6 PMTs (4 for fluorescence and 2 for laser light scatter).
- Coherent CUBE 40 milliwatt solid-state diode laser that emits red light at 638 nanometers and which is connected to a trigon that contains 3 fluorescent PMTs.
- Coherent CUBE 50 milliwatt solid-state diode laser that emits violet light at 405 nanometers and which is connected to a trigon that contains 2 fluorescent PMTs.
- Coherent Compass 25 milliwatt diode-pumped solid-state laser that emits yellow light at 561 nanometers and which is connected to a trigon that contains 2 fluorescent PMTs.
- Coherent Compass 150 milliwatt diode-pumped solid-state laser that emits green light at 532 nanometers and which is connected to a trigon that contains 3 fluorescent PMTs.
Differences in sorter model performance
There are notable differences in performance between the disparate sorters in the facility that investigators that are sorting must be aware of. Known differences are detailed below. The take-home message should be that each model sorter in the facility has strengths and weaknesses that investigators should take into consideration when planning experiments that involve sorting.
FACSAria advantages:
- The FACSAria is a closed-cuvette sorter that offers an advantage in fluorescence sensitivity over jet-in-air sorters such as the MoFlos. The fluorescence sensitivity of the FACSArias are equal to that observed on the bench top analyzer flow cytometers which also use a closed cuvette for fluorescence detection.
FACSAria disadvantages:
- The FACSArias cannot sort as fast as the MoFlos.
- The sort yield (recovery) is lower on the FACSAria as compared to the MoFlos'. The FACSAria's yield is significantly reduced, due to aborted events, in its upper speeds (events/second) as compared to the MoFlos'.
- The FACSAria's available nozzle sizes (70 or 100μ, with a 130μ nozzle available as 'special order') are not nearly as broad a range as the MoFlos', which is 50-200μ, with company plans to reintroduce a 400μ nozzle, so the choice of sorting very large cells (particles) does not exist on FACSArias.
- Due to the open architecture of the MoFlos' design, the FACSArias are not as flexible in their configuration.
- Persons working with Zebrafish should know that the FACSAria can discern only 3 of the 5 major Zebrafish embryo laser light-scatter populations that are observed on the MoFlos.
As stated previously, the take-home message is that each model sorter in the facility has strengths and weaknesses that investigators must take into consideration when planning experiments that involve sorting.
iCys - Laser Scanning Cytometry (LSC)
Introduction In the late 1990s, LSC has been developed that is capable of quantifying fluorescent and chromatic events in cells and tissues. LSC is a combination of microscopy and cytometry, all the principle (fluidic, optic and electronic) of flow cytometry basically can apply to laser scanning cytometry, except the fluidic is substituted by a moving stage of microscope. Since the data are acquired by microscopy lens, LSC provides not only quantitative ability of flow cytometry but also morphological images of microscopy.
Flow Cytometry Core Facility at Einstein recently acquired iCys equipped with a 405nm violet diode laser, a 488nm Argon laser and a 633nm Helium Neon laser from CompuCyte.
Below is the instrument and the optical configuration of our iCys.

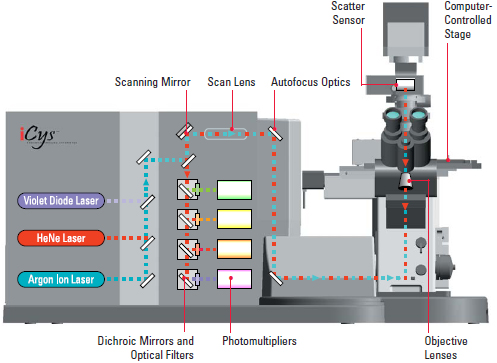
6 sensors are installed in this iCys including 2 scatter sensors and 4 photomultipliers. The table below shows some fluorescent and chromatic dyes that can be detected by iCys.
| Laser Excitation |
Wavelength Band |
Typical Fluorescent Dyes |
Typical Fhromatic Dyes |
| 405 nm |
430 - 470 nm (blue) |
DAPI, Hoechst33342, Pacific Blue, Quantum Dots |
DAB, Permanent red, Noav Red |
| 515 - 545 nm (green) |
Quantum Dots |
|
| 565 - 595 nm (orange) |
Pacific Orange, Quantum Dots |
|
| 650 - 800 nm (long red) |
Quantum Dots |
|
| 488 nm |
515 - 545 nm (green) |
FITC, Alex488, Syto 16, YoPro |
DAB, Permanent red, Nova Red, AEC |
| 565 - 595 nm (orange) |
PE, Mitoshift |
|
| 650 - 800 nm (long red) |
Pi, Pe-Cy5 |
|
| 633 nm |
650 - 800 nm (long red) |
Cy5, Alex647, Alex633, APC, DRAQ5 |
Hematoxinlin, BCIP |
Advantages of using LSC LSC offers the following advantages over flow cytometry:
- Adherent cells could be examined in the culture plates (see the carrier section below) without trypsinization or scraping allowing the analysis with minimal disturbance of the cells;
- Time-resolved events in individual cells can be measured;
- Morphological images could be obtained at the same time of data acquisition;
- Subcellular localization of fluorochrome could be visualized;
- The sample could be re-analyzed or even re-stained;
- Retention of tissue architecture allows evaluation of cell populations;
- Hypocellular samples (fine needle aspirate) could be measured in multiparametric manner;
- Addition parameters (maximal pixel, peripheral integral, etc) are available to enhance the analysis. For example, besides G0/G1, S and G2/M phases typically resolved by propidium iodide in flow cytometry, two more populations, M-mitotic and post-mitotic could be separated by using the maximal pixel parameter with the same stain.
Carrier Cells could be analyzed in the following carrier:
- Microtiter plates. In general, microplates contain cellular or DNA samples. CompuCyte recommends the following types of microplates:
96-well, plastic (such as Whatman cat# 7716-2380)
96-well, glass, skirtless (such as Whatman #7716-2370)
96-well, glass, skirted (such as Whatman #7716-2375)
48-well, tissue culture-treated (such as BD Falcon #353078)
24-well, tissue culture-treated (such as BD Falcon #353047)
12-well, tissue culture-treated (such as BD Falcon #353043)
6-well, tissue culture-treated (such as BD Falcon #353046)
384-well, optical quality plastic (such as Whatman #7706-2103)
384-well, optical quality plastic (such as Greiner #781096)
- Standard slide for both tissue and cellular samples.
- 35mm x 10mm culture dishes (such as Corning #430165).
Applications Below are some of the applications listed in the CompuCyte website or you may click into individual link to check out the details:
Using the LSC Individual who want to explore the instrument and to apply the technology in the research is encouraged to talk to the facility staffs.