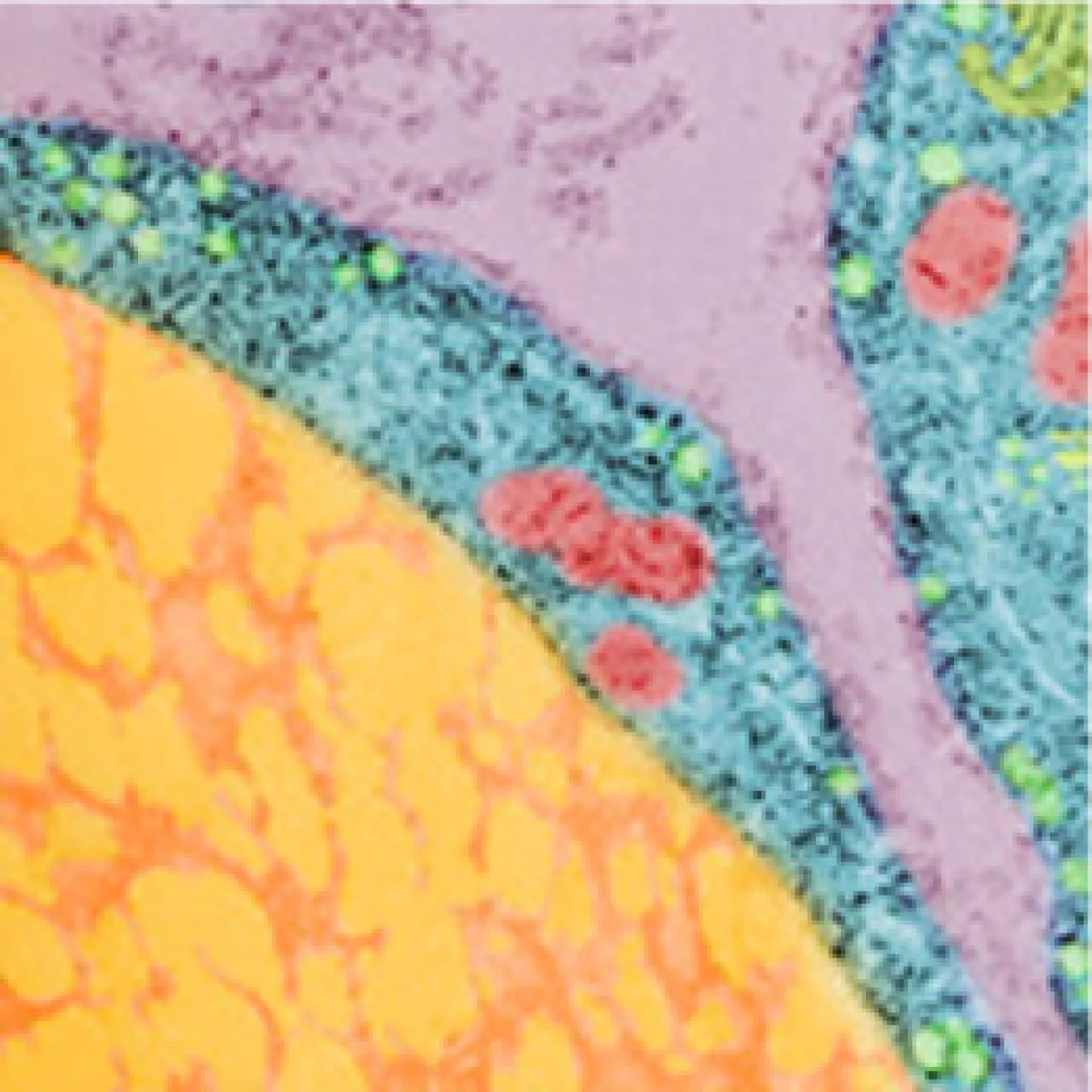
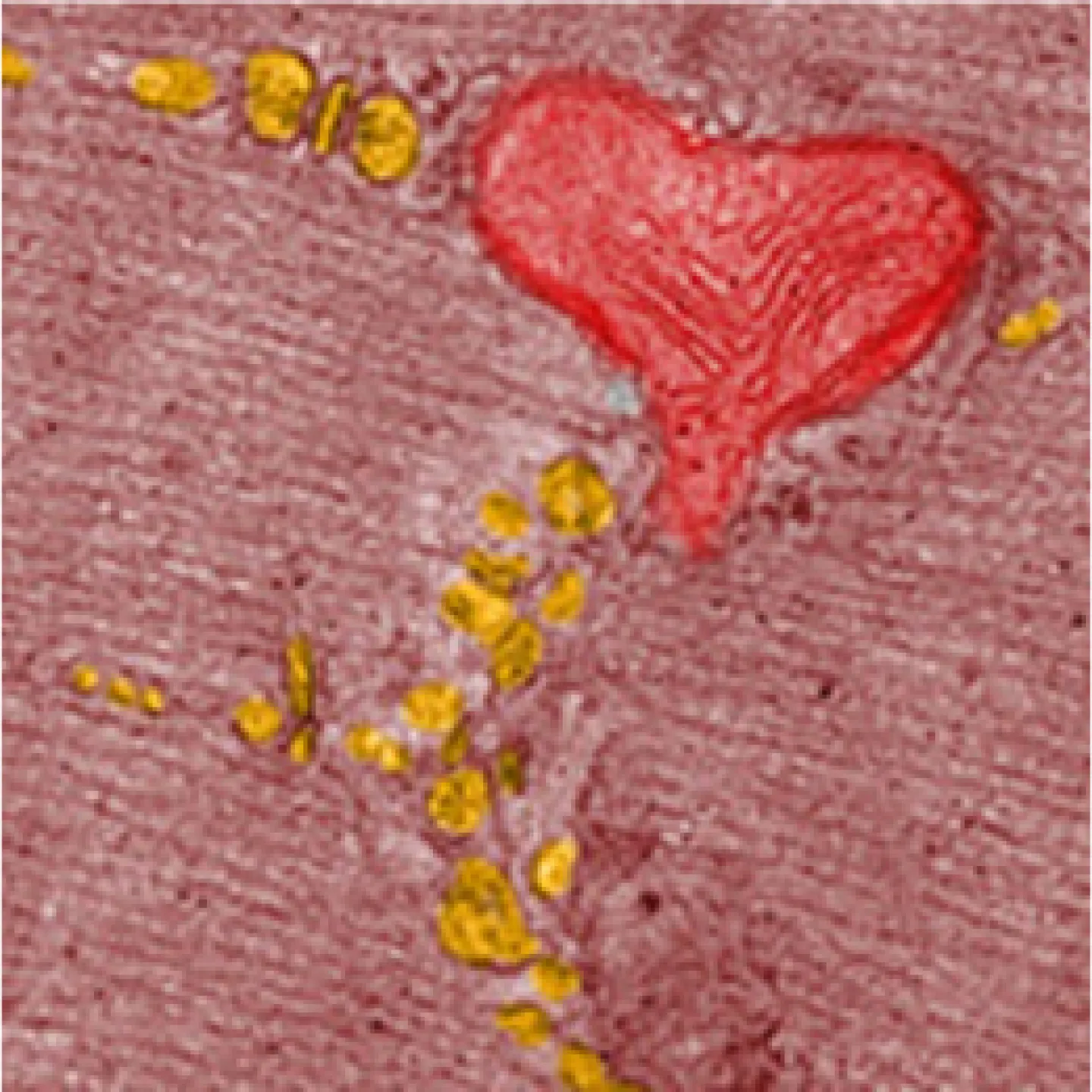
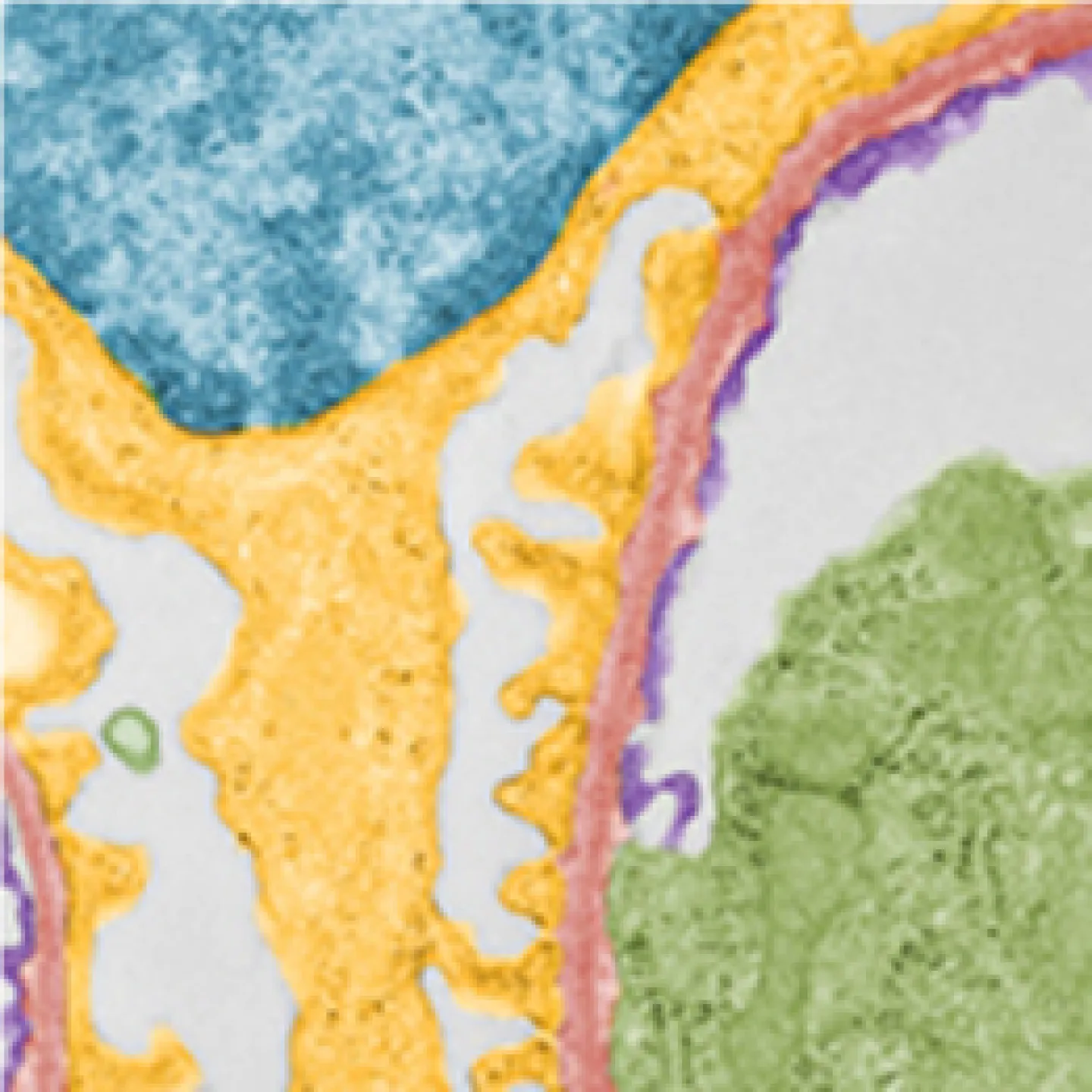
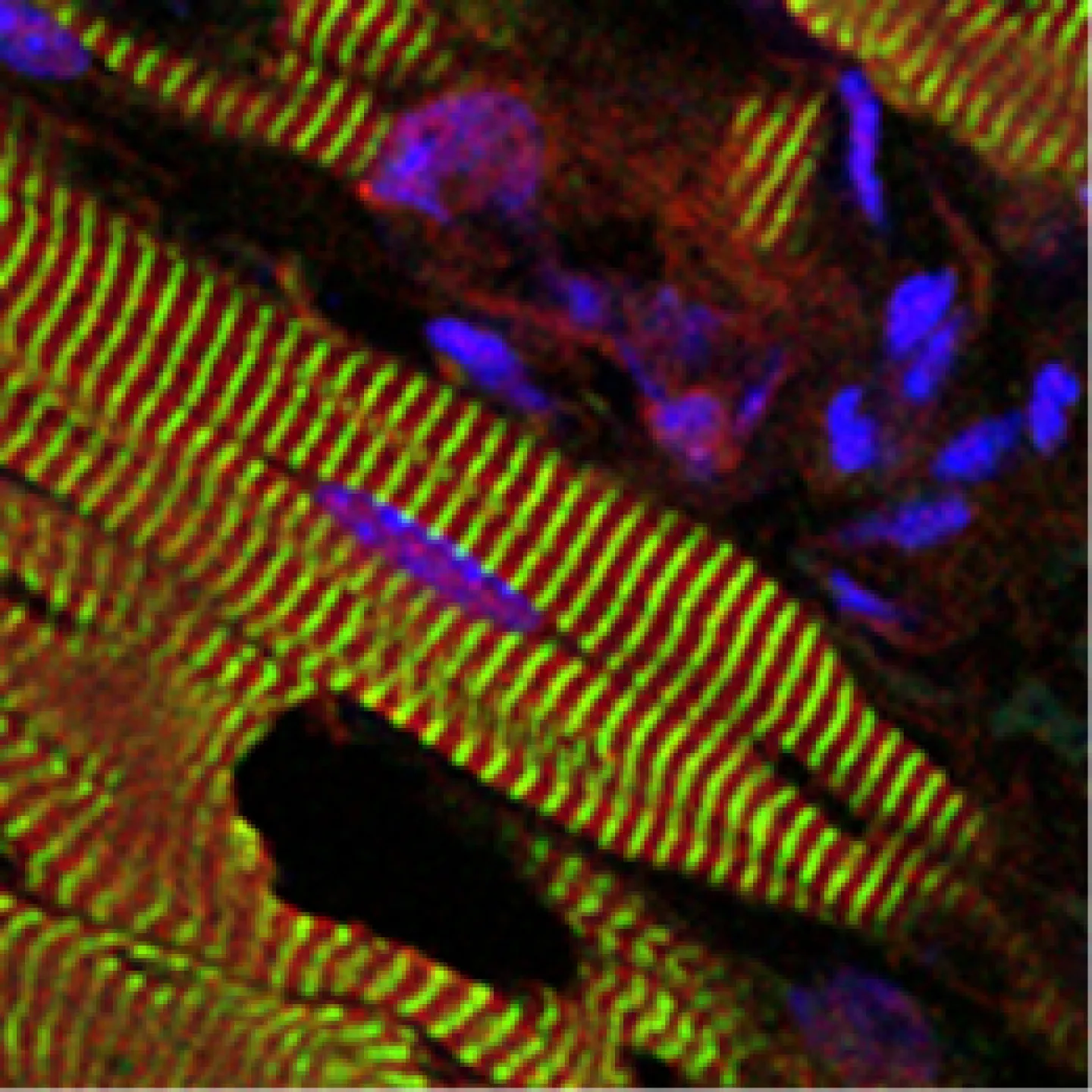
The Analytic Imaging Facility (AIF) has three transmission electron microscopes, all located in Forchheimer 633, as well as a scanning electron microscope. AIF can prepare the samples on your behalf.
Transmission Electron Microscopes (TEM)
Transmission electron microscopes (TEM) transmit a beam of electrons through an ultra-thin specimen, providing high resolution/high contrast detailed images.
Attention: The NIH requires that all publications using data from this instrument must list SIG # 1S10OD016214-01A1 in the Acknowledgments.
Microscope Parameters
- 120kV Transmission Electron Microscope
- LaB6 filament
- High contrast objective lens
Image Detection
- Gatan ClearView 16-megapixel CMOS camera
Additional Features
- High-tilt specimen holder
- Serial EM for Electron Tomography
- Mosaic stitching
- Gatan digital micrograph
Microscope Parameters
- 120kV Transmission Electron Microscope
- Tungsten filament
Image Detection
- -Gatan Orius 4-megapixel CCD camera
Additional Features
- Gatan digital micrograph
Please note: This microscope is used for low-dose imaging of vitreous-ice embedded samples
Microscope Parameters
- 200 kV transmission electron microscope
- LaB6 filament
- Low Dose mode for cryoEM
Image Detection
- TVIPS F-415 16-megapixel CCD camera
Additional Features
- High-tilt specimen holders
- Cryo specimen holders
- EM Menu4 acquisition software
- SerialEM for electron tomography
Scanning Electron Microscope
Scanning electron microscopes (SEM) use a focused beam of electrons to scan the sample, providing information about the sample's surface topography and composition.
Microscope Parameters
- 30 kV scanning electron microscope
- Field emission electron source
Image Detection
- Secondary and backscatter electrons, STEM
Additional Features
- Surface imaging
- Z contrast imaging
- Atlas 5 Array Tomography for 3D modeling
Specimen Preparation for Electron Microscopy
The AIF staff offers full-service sample preparation for many standard and customized EM techniques.
- You must speak with a member of the AIF staff before bringing samples for processing.
- Sample processing is scheduled to begin Wednesday mornings.
- AIF will provide the initial fix for your experiment.
- Pathogenic or infectious agents will only be accepted after primary fixation.
- Please use AIFs standard, recommended protocols for mammalian samples below.
- Embedding utilizing epoxy or acrylic resins at ambient or low temperatures
- Thin Sectioning with a Leica EM UC7, Leica UC6 or Leica Artos Ultramicrotome
- Array tomography for 3D reconstruction
- Immunogold Labeling following pre or post embedding protocols
- Cryoultramicrotomy using a Leica UC6 cryoultramicrotome
- Tokyasu immunogold labeling of ultrathin cryo sections
- Negative Staining of viruses, bacteria, endosomes, etc.
- Critical Point Drying using a Tousimis Samdri 790 Critical Point Dryer
- Sputter Coating using a EMS 150TES coating unit
- High Pressure Freezing using a Bal-Tec HPM-010 High Pressure Freezing Machine
- Freeze Substitution and Low Temperature Embedding in Leica EM AFS2 Freeze Substitution Unit
- Plunge Freezing of macromolecules with a FEI Mark IV Vitrobot
- Sacrifice your animal. Sacrifice one animal at a time!
- Quickly excise the tissue of interest.
- Place immediately into a Petri dish containing 2% paraformaldehyde + 2.5% gluteraldehyde in 0.1M Cacodylate buffer. The AIF staff will provide this for you.
- Cut tissue into small cubes. The cubes must have one dimension that is 1mm, the other dimensions may be slightly larger.
- Place 5 or 6 tissue cubes into a labeled vial containing fresh 2% paraformaldehyde + 2.5% gluteraldehyde in 0.1M Cacodylate buffer.
- Fix tissue for 60 minutes at room temperature.
- If it is early enough, bring tissue to AIF, room 639 in fixative. Be sure to speak to someone before leaving tissue.
- If it is the end of the day, replace fixative with 0.1M Cacodylate buffer and store overnight at 4° (in the fridge).
- Bring tissue (CUT UP CUBES) to AIF, room f639, in buffer. Be sure to speak to someone before leaving tissue.
- Grow your cells in plastic Petri dishes. We will accept any size dish but prefer 60mm. We also prefer duplicate dishes.
- Do fixation protocol one dish at a time! Do not let cells dry out!
- Rinse cells for 1 minute with serum free media at the incubation temperature.
- Pipette off the serum free media.
- Immediately add 2% paraformaldehyde + 2.5% gluteraldehyde in 0.1M Cacodylate buffer at room temperature. Gently add this from the edge of the dish. The AIF staff will provide this solution for you.
- Fix dish for 20 to 30 minutes at room temperature.
- Bring sample to AIF, room f639, in fixative. Be sure to speak to someone before leaving cells.
- Sacrifice your animal. Sacrifice one animal at a time!
- Quickly excise the tissue of interest.
- Place immediately into a Petri dish containing 4% Paraformaldehyde + 0.1%gluteraldehyde in 0.1M Cacodylate buffer. The AIF staff will provide this for you.
- Cut tissue into small cubes. The cubes must have one dimension that is 1mm, the other dimensions may be slightly larger.
- Place 5 or 6 tissue cubes into a labeled vial containing fresh 4% Paraformaldehyde, 0.1%gluteraldehyde in 0.1M Cacodylate buffer.
- Fix tissue for 60 minutes at room temperature.
- If it is early enough, bring tissue to AIF, room f639, in fixative. Be sure to speak to someone before leaving tissue!
- If it is the end of the day, replace fixative with 0.1M Cacodylate buffer and store overnight at 4° (in the fridge).
- Bring tissue (CUT UP CUBES) to AIF, room f639, in buffer. Be sure to speak to someone before leaving tissue!
- Grow your cells in plastic Petri dishes. We will accept any size dish, but prefer 60mm. We also prefer duplicate dishes.
- Do fixation protocol one dish at a time! Do not let cells dry out!
- Rinse cells for 1 minute with serum free media at the incubation temperature.
- Pipette off the serum free media.
- Immediately add 4% Paraformaldehyde, 0.1%gluteraldehyde in 0.1M Cacodylate buffer at room temperature. Gently add this from the edge of the dish. The AIF staff will provide this solution for you.
- Fix dish for 20 to 30 minutes at room temperature.
- Bring sample to AIF, room f639, in fixative. Be sure to speak to someone before leaving cells!
- Sacrifice your animal. Sacrifice one animal at a time!
- Quickly excise the tissue of interest.
- Place immediately into a Petri dish containing 2.5% gluteraldehyde in 0.1M Cacodylate buffer with 5mM MgCl + 0.2M sucrose. The AIF staff will provide this for you.
- Cut tissue into areas of interest. Do not hold an area of interest with forceps! This will show as mechanical damage to your tissue in the SEM.
- Place tissue of interest into a labeled vial containing fresh 2.5% gluteraldehyde in 0.1M Cacodylate buffer.
- Fix tissue for 60 minutes at room temperature.
- If it is early enough, bring tissue to AIF, room 639 in fixative. Be sure to speak to someone before leaving tissue!
- If it is the end of the day, replace fixative with 0.1M Cacodylate buffer and store overnight at 4° (in the fridge).
- Bring tissue to AIF, room f639, in buffer. Be sure to speak to someone before leaving tissue!
- Grow your cells on glass 13 mm round coverslips in plastic Petri dishes. The AIF staff will provide these for you. You must use the coverslips provided by the AIF. They must be cleaned and sterilized before plating out your cells.
- Do fixation protocol one dish at a time! Do not let cells dry!
- Give your cells a serum free media rinse. This washes out any proteins that may be in the dish.
- Pipette off the serum free media.
- Immediately add 2.5% gluteraldehyde in 0.1M Cacodylate buffer with MgCl + sucrose. Gently add this from the edge of the dish. The AIF staff will provide this solution for you.
- Fix dish for 20 to 30 minutes at room temperature.
- Bring sample to AIF, room f639, in fixative. Be sure to speak to someone before leaving cells!