Cayuse Handbook
Download FOA application package on
Cayuse server
Most FOAs are already in the Cayuse system.
If a particular FOA does not appear on the
pop-up list after clicking "Create
Proposal," go into the Opportunities tab and
add it. Click on "Retrieve Opportunity" and
type in the FOA Number (e.g.,
RFA-AG-12-000). If it cannot be added,
please contact the Office of Grant Support
(X3643) for assistance.
The proposal can be started in either
Proposals S2S or Cayuse SP. We recommend
building your proposal in Cayuse SP, but
proposals can also be paired after creation.
- Click on My Dashboard, and click
on Start New Proposal
- Select either grants.gov for the full
S2S application package or other for the
abbreviated package.
-
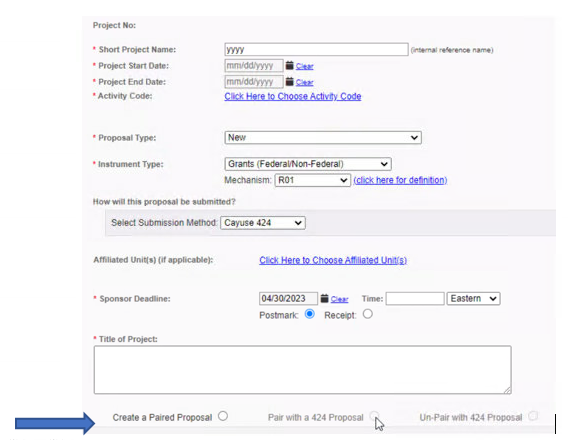
Fill out Sponsor Information and
General Information for your
proposal. All fields marked with a
red asterisk are required.
-
Complete the Short Project Name field.
(This is known as the Proposal Name field in Cayuse 424.)
Please follow the
naming conventions below, using
Einstein’s guidelines (It’s an
internal title):
- Competitive grants: PI’s
last name/Grant
Mechanism/Deadline (e.g.,
Smith/R01/2-5-16)
- Non-competing RPPRs/Progress
reports: NC-PIs last
name/Grant Mechanism/Deadline
(e.g., NC-Smith/R01/2-15-16)
- Click save at the bottom of the page
It is also possible to copy an existing
proposal. When copying a cayuse proposal,
the following information is copied:
- General tab information
- Approving units information
Pairing/Linking
Upon filling out General and Sponsor
Information when creating your proposal, you
will see an option to pair your Cayuse SP
proposal with a Cayuse 424 proposal. During
proposal creation, only one of these options
is active: Create a Paired Proposal.
Upon saving this page, Pair with a 424
Proposal will also be active.